A Comprehensive Roadmap Towards the Generation of an Influenza B Reporter Assay Using a Single DNA Polymerase-Based Cloning of the Reporter RNA Construct
- PMID: 35694292
- PMCID: PMC9174941
- DOI: 10.3389/fmicb.2022.868367
A Comprehensive Roadmap Towards the Generation of an Influenza B Reporter Assay Using a Single DNA Polymerase-Based Cloning of the Reporter RNA Construct
Abstract
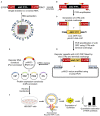
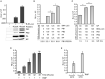
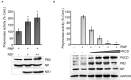
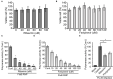
The mini-genome reporter assay is a key tool for conducting RNA virus research. However, procedural complications and the lack of adequate literature pose a major challenge in developing these assay systems. Here, we present a novel, yet generic and simple, cloning strategy for the construction of an influenza B virus reporter RNA template and describe an extensive standardization of the reporter RNP/polymerase activity assay for monitoring viral RNA synthesis in an infection-free setting. Using this assay system, we showed for the first time the effect of viral protein NS1 and host protein kinase C delta (PKCD) on influenza B virus RNA synthesis. In addition, the assay system showed promising results in evaluating the efficacy of antiviral drugs targeting viral RNA synthesis and virus propagation. Together, this work offers a detailed protocol for the standardization of the influenza virus minigenome assay and an excellent tool for screening of host factors and antivirals in a fast, user-friendly, and high-throughput manner.
Keywords: antiviral screening; influenza B; polymerase activity assay; reporter construct; ribonucleoprotein.
Copyright © 2022 Kedia, Banerjee and Mondal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- CDC (2021a). U. S. Influenza Surveillance: Purpose and Methods | CDC. Available online at: https://www.cdc.gov/flu/weekly/overview.htm (accessed November 2, 2021).
-
- CDC (2021b). Flu Symptoms & Complications | CDC. Available online at: https://www.cdc.gov/flu/symptoms/symptoms.htm (accessed November 2, 2021).
LinkOut - more resources
Full Text Sources
Research Materials

