Discovery of a phase-separating small molecule that selectively sequesters tubulin in cells
- PMID: 35694339
- PMCID: PMC9116451
- DOI: 10.1039/d1sc07151c
Discovery of a phase-separating small molecule that selectively sequesters tubulin in cells
Abstract
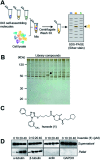
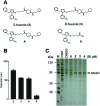
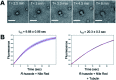
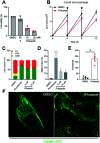
Phase-separated membraneless organelles or biomolecular condensates play diverse functions in cells, however recapturing their characteristics using small organic molecules has been a challenge. In the present study, cell-lysate-based screening of 843 self-assembling small molecules led to the discovery of a simple organic molecule, named huezole, that forms liquid droplets to selectively sequester tubulin. Remarkably, this small molecule enters cultured human cells and prevents cell mitosis by forming tubulin-concentrating condensates in cells. The present study demonstrates the feasibility of producing a synthetic condensate out of non-peptidic small molecules for exogenous control of cellular processes. The modular structure of huezole provides a framework for designing a class of organelle-emulating small molecules.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures