Long Term Metabolic Effects of Sacubitril/Valsartan in Non-Diabetic and Diabetic Patients With Heart Failure Reduced Ejection Fraction: A Real Life Study
- PMID: 35694400
- PMCID: PMC9174635
- DOI: 10.3389/fphys.2022.897109
Long Term Metabolic Effects of Sacubitril/Valsartan in Non-Diabetic and Diabetic Patients With Heart Failure Reduced Ejection Fraction: A Real Life Study
Abstract
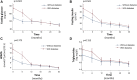
Sacubitril/Valsartan (sac/val) has improved clinical prognosis in patients affected by heart failure (HF) with reduced ejection fraction (HFrEF). HF and type 2 diabetes mellitus (T2DM) frequently coexist, with a prevalence of T2DM of 35%-40% in patients with HF. T2DM is the third co-morbidities in patients with HF and a strong independent risk factor for the progression of HF. In a post hoc analysis of PARADIGM-HF, improved glycemic control was shown in patients with T2DM and HFrEF receiving sac/val compared to enalapril at 12 months of follow-up. The aim of the present study was to evaluate, in a series of repeated observations in 90 HFrEF patients, the long term effect of sac/val treatment on renal function, glycometabolic state and insulin sensitivity parameters, according to diabetic status. We studied 90 patients (74 men and 16 women, mean age 68 ± 10 years, 60 diabetics and 30 non-diabetics) suffering from HFrEF and still symptomatic despite optimal pharmacological therapy. Patients with left ventricular ejection fraction (LVEF) <35% and II-III NYHA functional class were enrolled. All patients underwent clinical-instrumental and laboratory determinations and Minnesota Living with HF Questionnaire (MLHFQ) every 6 months until 30 months to evaluate benefits and adverse events. After 30 months follow-up, we observed a significant improvement in glycometabolic parameters including HbA1c, fasting glucose and insulin, insulin-like growth factor-1 (IGF-1), HOMA index, and LDL cholesterol. Moreover, renal function, NTpro-BNP levels and echocardiographic parameters significantly improved. In diabetic patients a significant reduction in use of oral antidiabetic drugs and insulin was observed after 30 months of sac/val treatment. In the whole population, multivariate analysis shows that the evolution of cardiac index (CI) was significantly associated to simultaneous changes in HOMA, IGF-1 and visit; per each visit and for 1 ng/ml increase in IGF-1 there was an increase in CI of 64.77 ml/min/m2 (p < 0.0001) and 0.98 ml/min/m2 (p = 0.003), respectively, whereas 1 point increase in HOMA was associated with a -7.33 ml/min/m2 (p = 0.003) reduction in CI. The present data confirm persistent metabolic improvement in patients with HFrEF after treatment with sac/val and highlights its potential therapeutical role in patients with metabolic comorbidities.
Keywords: HbA1c; cardiac index; global longitudinal strain; heart failure with reduced ejection fraction; sacubitril/valsartan; type 2 diabetes mellitus.
Copyright © 2022 Armentaro, D’Arrigo, Miceli, Cassano, Perticone, Maio, Marra, Arturi, Cittadini, Tripepi, Sesti and Sciacqua.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Armentaro G., D’Arrigo G., Magurno M., Toscani A. F., Condoleo V., Miceli S., et al. (2021). Impact of Sacubitril/Valsartan on Clinical and Echocardiographic Parameters in Heart Failure Patients with Reduced Ejection Fraction: Data from a Real Life 2-year Follow-Up Study. Front. Pharmacol. 12, 733475. 10.3389/fphar.2021.733475 - DOI - PMC - PubMed
-
- Badano L. P., Kolias T. J., Muraru D., Abraham T. P., Aurigemma G., Edvardsen T., et al. (2018). Standardization of Left Atrial, Right Ventricular, and Right Atrial Deformation Imaging Using Two-Dimensional Speckle Tracking Echocardiography: a Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. Cardiovasc Imaging 19, 591–600. 10.1093/ehjci/jey042 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

