Targeting Stress Erythropoiesis Pathways in Cancer
- PMID: 35694408
- PMCID: PMC9174937
- DOI: 10.3389/fphys.2022.844042
Targeting Stress Erythropoiesis Pathways in Cancer
Abstract
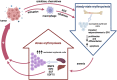
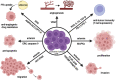
Cancer-related anemia (CRA) is a common multifactorial disorder that adversely affects the quality of life and overall prognosis in patients with cancer. Safety concerns associated with the most common CRA treatment options, including intravenous iron therapy and erythropoietic-stimulating agents, have often resulted in no or suboptimal anemia management for many cancer patients. Chronic anemia creates a vital need to restore normal erythropoietic output and therefore activates the mechanisms of stress erythropoiesis (SE). A growing body of evidence demonstrates that bone morphogenetic protein 4 (BMP4) signaling, along with glucocorticoids, erythropoietin, stem cell factor, growth differentiation factor 15 (GDF15) and hypoxia-inducible factors, plays a pivotal role in SE. Nevertheless, a chronic state of SE may lead to ineffective erythropoiesis, characterized by the expansion of erythroid progenitor pool, that largely fails to differentiate and give rise to mature red blood cells, further aggravating CRA. In this review, we summarize the current state of knowledge on the emerging roles for stress erythroid progenitors and activated SE pathways in tumor progression, highlighting the urgent need to suppress ineffective erythropoiesis in cancer patients and develop an optimal treatment strategy as well as a personalized approach to CRA management.
Keywords: anemia; cancer; erythroid progenitors; erythropoietin; stress erythropoiesis.
Copyright © 2022 Vignjević Petrinović, Jauković, Milošević, Bugarski and Budeč.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Acs G., Zhang P. J., McGrath C. M., Acs P., McBroom J., Mohyeldin A., et al. (2003). Hypoxia-inducible Erythropoietin Signaling in Squamous Dysplasia and Squamous Cell Carcinoma of the Uterine Cervix and its Potential Role in Cervical Carcinogenesis and Tumor Progression. Am. J. Pathology 162, 1789–1806. 10.1016/S0002-9440(10)64314-3 - DOI - PMC - PubMed
-
- Aguilar C., Aguilar C., Lopez-Marure R., Jiménez-sánchez A., Rocha-Zavaleta L. (2014). Co-stimulation with Stem Cell Factor and Erythropoietin Enhances Migration of C-Kit Expressing Cervical Cancer Cells through the Sustained Activation of ERK1/2. Mol. Med. Rep. 9, 1895–1902. 10.3892/mmr.2014.2044 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

