Detangling the Crosstalk Between Ascaris, Trichuris and Gut Microbiota: What´s Next?
- PMID: 35694539
- PMCID: PMC9174645
- DOI: 10.3389/fcimb.2022.852900
Detangling the Crosstalk Between Ascaris, Trichuris and Gut Microbiota: What´s Next?
Abstract
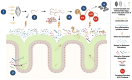
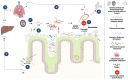
Helminth infections remain a global public health issue, particularly in low- and middle-income countries, where roundworms from theTrichuris and Ascaris genera are most prevalent. These geohelminths not only impact human health but most importantly also affect animal well-being, in particular the swine industry. Host-helminth parasite interactions are complex and at the same time essential to understand the biology, dynamics and pathophysiology of these infections. Within these interactions, the immunomodulatory capacity of these helminths in the host has been extensively studied. Moreover, in recent years a growing interest on how helminths interact with the intestinal microbiota of the host has sparked, highlighting how this relationship plays an essential role in the establishment of initial infection, survival and persistence of the parasite, as well as in the development of chronic infections. Identifying the changes generated by these helminths on the composition and structure of the host intestinal microbiota constitutes a field of great scientific interest, since this can provide essential and actionable information for designing effective control and therapeutic strategies. Helminths like Trichuris and Ascaris are a focus of special importance due to their high prevalence, higher reinfection rates, resistance to anthelmintic therapy and unavailability of vaccines. Therefore, characterizing interactions between these helminths and the host intestinal microbiota represents an important approach to better understand the nature of this dynamic interface and explore novel therapeutic alternatives based on management of host microbiota. Given the extraordinary impact this may have from a biological, clinical, and epidemiological public health standpoint, this review aims to provide a comprehensive overview of current knowledge and future perspectives examining the parasite-microbiota interplay and its impact on host immunity.
Keywords: Ascaris; Trichuris; helminths; host-parasite interactions; microbiota.
Copyright © 2022 Castañeda, Paniz-Mondolfi and Ramírez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Guts within guts: the microbiome of the intestinal helminth parasite Ascaris suum is derived but distinct from its host.Microbiome. 2022 Dec 16;10(1):229. doi: 10.1186/s40168-022-01399-5. Microbiome. 2022. PMID: 36527132 Free PMC article.
-
Helminth-microbiota cross-talk - A journey through the vertebrate digestive system.Mol Biochem Parasitol. 2019 Oct;233:111222. doi: 10.1016/j.molbiopara.2019.111222. Epub 2019 Sep 18. Mol Biochem Parasitol. 2019. PMID: 31541662 Review.
-
Helminth parasites in pigs: new challenges in pig production and current research highlights.Vet Parasitol. 2011 Aug 4;180(1-2):72-81. doi: 10.1016/j.vetpar.2011.05.029. Epub 2011 May 27. Vet Parasitol. 2011. PMID: 21684689 Review.
-
Helminth-Bacterial Interactions: Cause and Consequence.Trends Immunol. 2018 Sep;39(9):724-733. doi: 10.1016/j.it.2018.06.002. Epub 2018 Jun 22. Trends Immunol. 2018. PMID: 29941203 Review.
-
Helminths, polyparasitism, and the gut microbiome in the Philippines.Int J Parasitol. 2020 Mar;50(3):217-225. doi: 10.1016/j.ijpara.2019.12.008. Epub 2020 Mar 3. Int J Parasitol. 2020. PMID: 32135180
Cited by
-
In Vitro Filaricidal Properties of Aqueous Extracts of Combretum nigricans (Combretaceae) on Onchocerca ochengi (Onchocercidae).J Parasitol Res. 2024 Jan 31;2024:2119056. doi: 10.1155/2024/2119056. eCollection 2024. J Parasitol Res. 2024. PMID: 38328477 Free PMC article.
-
Nematode-bacteria interactions in bovine parasitic otitis.Rev Bras Parasitol Vet. 2024 Dec 20;33(4):e019024. doi: 10.1590/S1984-29612024081. eCollection 2024. Rev Bras Parasitol Vet. 2024. PMID: 39774744 Free PMC article.
-
Metabarcoding of bacteria and parasites in the gut of Apodemus agrarius.Parasit Vectors. 2022 Dec 23;15(1):486. doi: 10.1186/s13071-022-05608-w. Parasit Vectors. 2022. PMID: 36564849 Free PMC article.
-
Opisthorchis viverrini, Clonorchis sinensis and Opisthorchis felineus liver flukes affect mammalian host microbiome in a species-specific manner.PLoS Negl Trop Dis. 2023 Feb 13;17(2):e0011111. doi: 10.1371/journal.pntd.0011111. eCollection 2023 Feb. PLoS Negl Trop Dis. 2023. PMID: 36780567 Free PMC article.
-
Parasite-microbiota interactions: a pathway to innovative interventions for Chagas disease, leishmaniasis, and ascariasis.Future Microbiol. 2025 Feb;20(2):149-161. doi: 10.1080/17460913.2024.2431417. Epub 2024 Nov 22. Future Microbiol. 2025. PMID: 39574234 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

