The Microbiota and It's Correlation With Metabolites in the Gut of Mice With Nonalcoholic Fatty Liver Disease
- PMID: 35694542
- PMCID: PMC9186341
- DOI: 10.3389/fcimb.2022.870785
The Microbiota and It's Correlation With Metabolites in the Gut of Mice With Nonalcoholic Fatty Liver Disease
Erratum in
-
Corrigendum: The Microbiota and It's Correlation With Metabolites in the Gut of Mice With Nonalcoholic Fatty Liver Disease.Front Cell Infect Microbiol. 2022 Jul 5;12:972118. doi: 10.3389/fcimb.2022.972118. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35865827 Free PMC article.
Abstract
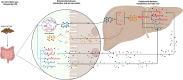
In recent years, nonalcoholic fatty liver disease (NAFLD) has become the most common liver disease in the world. As an important model animal, the characteristics of gut microbiota alteration in mice with NAFLD have been studied but the changes in metabolite abundance in NAFLD mice and how the gut microbiota affects these intestinal metabolites remain unclear. In this experiment, a mouse model for NAFLD was established by a high-fat diet. The use of 16S rDNA technology showed that while there were no significant changes in the alpha diversity in the cecum of NAFLD mice, the beta diversity changed significantly. The abundance of Blautia, Unidentified-Lachnospiraceae, Romboutsia, Faecalibaculum, and Ileibacterium increased significantly in NAFLD mice, while Allobaculum and Enterorhabdus decreased significantly. Amino acids, lipids, bile acids and nucleotide metabolites were among the 167 significantly different metabolites selected. The metabolic pathways of amino acids, SFAs, and bile acids were significantly enhanced, while the metabolic pathways of PUFAs, vitamins, and nucleotides were significantly inhibited. Through correlation and MIMOSA2 analysis, it is suggested that gut microbiota does not affect the changes of lipids and bile acids but can reduce thiamine, pyridoxine, and promote L-phenylalanine and tyramine production. The findings of this study will help us to better understand the relationship between gut microbiota and metabolites in NAFLD.
Keywords: 16SrDNA; MIMOSA2; metabonomics; mice; nonalcoholic fatty liver disease (NAFLD).
Copyright © 2022 Gu, Zhou, Yu, He, He, Luo, Xiao, Yang, Zhao, Li, Shen, Han, Cao, Zuo, Deng, Yan, Ren, Zhao and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Arao Y., Kawai H., Kamimura K., Kobayashi T., Nakano O., Hayatsu M., et al. . (2020). Effect Of Methionine/Choline-Deficient Diet and High-Fat Diet-Induced Steatohepatitis on Mitochondrial Homeostasis in Mice. Biochem. Biophys. Res. Commun. 527, 365–371. doi: 10.1016/j.bbrc.2020.03.180 - DOI - PubMed
-
- Araujo T. R., Freitas I. N., Vettorazzi J. F., Batista T. M., Santos-Silva J. C., Bonfleur M. L., et al. . (2016). Benefits Of L-Alanine Or L-Arginine Supplementation Against Adiposity and Glucose Intolerance in Monosodium Glutamate-Induced Obesity. Eur. J. Nutr. 56, 2069–2080. doi: 10.1007/s00394-016-1245-6 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

