Vasopressin for Post-kidney Transplant Hypotension
- PMID: 35694563
- PMCID: PMC9174042
- DOI: 10.1016/j.ekir.2022.03.035
Vasopressin for Post-kidney Transplant Hypotension
Abstract
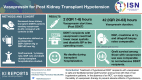
Introduction: Hypotension after deceased donor kidney transplant (DDKT) is a risk factor for delayed graft function (DGF) and poor graft survival (GS). We hypothesize that vasopressin use in hypotensive DDKT recipients (DDKTRs) to increase blood pressure (BP) reduces DGF rates and is safe without increasing mortality.
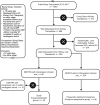
Methods: Group with vasopressin "study group" (n = 45) was defined as DDKTRs between 2012 and 2017 who required vasopressin for hypotension systolic BP (SBP) <120 mm Hg or diastolic BP (DBP) <60 mm Hg. DDKTRs with no-vasopressin "comparison group" (n = 90) were propensity score-matched DDKTRs between 2012 and 2017 without vasopressin use. Primary outcomes were GS, creatinine and allograft biopsy rate at 1 year, DGF rate, and death during transplant hospitalization.
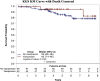
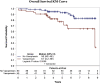
Results: Vasopressin group had lower mean maximum and minimum SBP and DBP in the operating room (OR). Median vasopressin start time post-DDKT was 2 hours (interquartile range [IQR] 1-6), and duration of use was 42 hours (IQR 24-63). DGF, creatinine at 1 year, and allograft biopsy rates were comparable. No deaths occurred during transplant hospitalization. Multivariable analysis did not find an effect of vasopressin use on GS.
Conclusion: Treatment of hypotensive DDKTRs with vasopressin is safe and facilitated similar graft function and survival with that of nonhypotensive patients. In the absence of a randomized control trial, our study supports the safety of vasopressin therapy to prevent the adverse effects of hypotension.
Keywords: deceased donor kidney transplant; delayed graft function; graft survival; vasopressin.
© 2022 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
LinkOut - more resources
Full Text Sources

