Insights into the recent progress in the medicinal chemistry of pyranopyrimidine analogs
- PMID: 35694689
- PMCID: PMC9133730
- DOI: 10.1039/d2md00076h
Insights into the recent progress in the medicinal chemistry of pyranopyrimidine analogs
Abstract
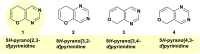
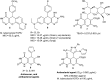
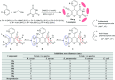
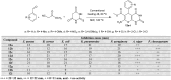
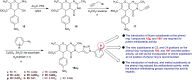
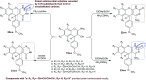
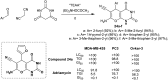
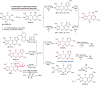
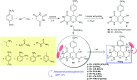
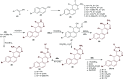
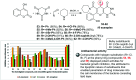
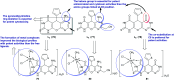
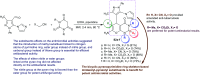
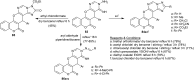
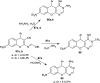
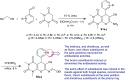
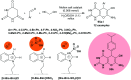
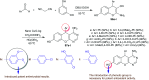
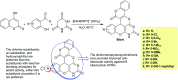
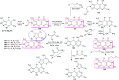
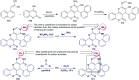
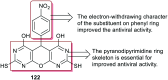
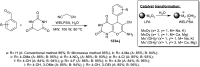
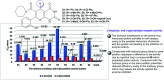
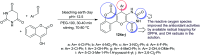
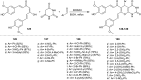
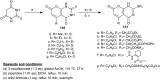
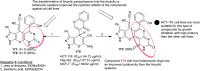
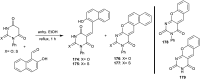
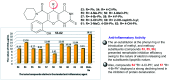
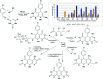
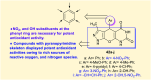
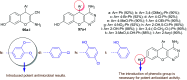
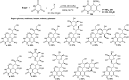
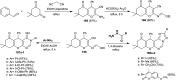
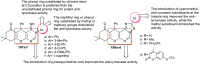
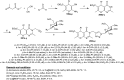
Heterocycles containing the pyranopyrimidine motif have attracted the interest of researchers in recent decades due to their ability to synthesize and explore at a large scale to explore the biological diversity. Therefore, this review highlights the biological characteristics and synthetic approaches adopted to prepare pyranopyrimidine analogs in the last five years. Several novel preparation procedures have been summarized to synthesize these compounds using ionic, basic, or nanocatalysts or catalyst-free conditions to obtain these compounds in good yields. Pyranopyrimidines could also be used as ligands in the preparation of metal complexes with increased biological potency. The different sections include the antimicrobial, antitubercular, antimalarial, antiviral "SARS-CoV-2 inhibitors", antidiabetic, antitumor, cytotoxic, antiinflammatory, antioxidant, anticoagulant, urease inhibitory activities, and tyrosine inhibitors. The results are discussed based on the structure-activity relationships (SARs) and the mechanism of action.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors state no conflict of interest.
Figures









Similar articles
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
-
A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives.Pharmacol Rep. 2017 Apr;69(2):281-295. doi: 10.1016/j.pharep.2016.11.007. Epub 2016 Nov 18. Pharmacol Rep. 2017. PMID: 28171830 Review.
-
Therapeutic Journey and Recent Advances in the Synthesis of Coumarin Derivatives.Mini Rev Med Chem. 2022;22(9):1314-1330. doi: 10.2174/1389557521666211116120823. Mini Rev Med Chem. 2022. PMID: 34784861 Review.
-
Triazole analogues as potential pharmacological agents: a brief review.Futur J Pharm Sci. 2021;7(1):106. doi: 10.1186/s43094-021-00241-3. Epub 2021 May 25. Futur J Pharm Sci. 2021. PMID: 34056014 Free PMC article. Review.
-
An insight into the medicinal perspective of synthetic analogs of indole: A review.Eur J Med Chem. 2019 Oct 15;180:562-612. doi: 10.1016/j.ejmech.2019.07.019. Epub 2019 Jul 11. Eur J Med Chem. 2019. PMID: 31344615 Review.
Cited by
-
Developments of pyridodipyrimidine heterocycles and their biological activities.Mol Divers. 2024 Apr;28(2):927-964. doi: 10.1007/s11030-023-10623-9. Epub 2023 Feb 25. Mol Divers. 2024. PMID: 36840839 Free PMC article. Review.
-
Recent progress in the chemistry of β-aminoketones.RSC Adv. 2022 Aug 31;12(38):24681-24712. doi: 10.1039/d2ra03864a. eCollection 2022 Aug 30. RSC Adv. 2022. PMID: 36128366 Free PMC article. Review.
-
Hybrids Diazine: Recent Advancements in Modern Antimicrobial Therapy.Curr Med Chem. 2024;31(19):2687-2705. doi: 10.2174/0929867330666230418104409. Curr Med Chem. 2024. PMID: 37073649 Review.
-
Synthetic strategies of heterocycle-integrated pyridopyrimidine scaffolds supported by nano-catalysts.RSC Adv. 2023 Apr 14;13(17):11600-11634. doi: 10.1039/d3ra00922j. eCollection 2023 Apr 11. RSC Adv. 2023. PMID: 37063723 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

