Natural history of a mouse model of X-linked myotubular myopathy
- PMID: 35694952
- PMCID: PMC9346535
- DOI: 10.1242/dmm.049342
Natural history of a mouse model of X-linked myotubular myopathy
Abstract
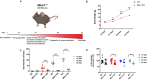
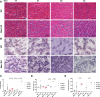
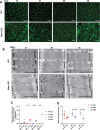
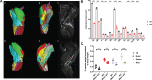
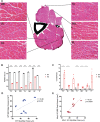
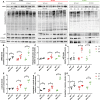
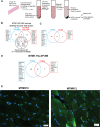
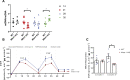
X-linked myotubular myopathy (XLMTM) is a severe monogenetic disorder of the skeletal muscle. It is caused by loss-of-expression/function mutations in the myotubularin (MTM1) gene. Much of what is known about the disease, as well as the treatment strategies, has been uncovered through experimentation in pre-clinical models, particularly the Mtm1 gene knockout mouse line (Mtm1 KO). Despite this understanding, and the identification of potential therapies, much remains to be understood about XLMTM disease pathomechanisms, and about the normal functions of MTM1 in muscle development. To lay the groundwork for addressing these knowledge gaps, we performed a natural history study of Mtm1 KO mice. This included longitudinal comparative analyses of motor phenotype, transcriptome and proteome profiles, muscle structure and targeted molecular pathways. We identified age-associated changes in gene expression, mitochondrial function, myofiber size and key molecular markers, including DNM2. Importantly, some molecular and histopathologic changes preceded overt phenotypic changes, while others, such as triad structural alternations, occurred coincidentally with the presence of severe weakness. In total, this study provides a comprehensive longitudinal evaluation of the murine XLMTM disease process, and thus provides a critical framework for future investigations.
Keywords: Mice; Muscle disease; Myotubular myopathy; Myotubularin; Natural history.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests J.J.D. has sponsored research agreements with Astellas Gene Therapy and Dynacure. None of the research in this study was performed as part of these agreements. For all other authors, there are no competing or financial interests.
Figures


References
-
- Acin-Perez, R., Benador, I. Y., Petcherski, A., Veliova, M., Benavides, G. A., Lagarrigue, S., Caudal, A., Vergnes, L., Murphy, A. N., Karamanlidis, G.et al. (2020). A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO J. 39, e104073. 10.15252/embj.2019104073 - DOI - PMC - PubMed
-
- Al-Qusairi, L., Weiss, N., Toussaint, A., Berbey, C., Messaddeq, N., Kretz, C., Sanoudou, D., Beggs, A. H., Allard, B., Mandel, J. L.et al. (2009). T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc. Natl. Acad. Sci. USA 106, 18763-18768. 10.1073/pnas.0900705106 - DOI - PMC - PubMed
-
- Al-Qusairi, L., Prokic, I., Amoasii, L., Kretz, C., Messaddeq, N., Mandel, J. L. and Laporte, J. (2013). Lack of myotubularin (MTM1) leads to muscle hypotrophy through unbalanced regulation of the autophagy and ubiquitin-proteasome pathways. FASEB J. 27, 3384-3394. 10.1096/fj.12-220947 - DOI - PubMed
-
- Annoussamy, M., Lilien, C., Gidaro, T., Gargaun, E., Che, V., Schara, U., Gangfuss, A., D'Amico, A., Dowling, J. J., Darras, B. T.et al. (2019). X-linked myotubular myopathy: A prospective international natural history study. Neurology 92, e1852-e1867. 10.1212/WNL.0000000000007319 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

