Neuroendocrine, inflammatory, and extracellular vesicle responses during the Navy Special Warfare Screener Selection Course
- PMID: 35695270
- PMCID: PMC9291410
- DOI: 10.1152/physiolgenomics.00184.2021
Neuroendocrine, inflammatory, and extracellular vesicle responses during the Navy Special Warfare Screener Selection Course
Abstract
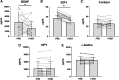
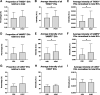
Military operational stress is known to increase adrenal hormones and inflammatory cytokines, while decreasing hormones associated with the anabolic milieu and neuroendocrine system. Less is known about the role of extracellular vesicles (EVs), a form of cell-to-cell communication, in military operational stress and their relationship to circulating hormones. The purpose of this study was to characterize the neuroendocrine, cytokine, and EV response to an intense. 24-h selection course known as the Naval Special Warfare (NSW) Screener and identify associations between EVs and cytokines. Blood samples were collected the morning of and following the NSW Screener in 29 men (18-26 yr). Samples were analyzed for concentrations of cortisol, insulin-like growth factor I (IGF-I), neuropeptide-Y (NPY), brain-derived neurotrophic factor (BDNF), α-klotho, tumor necrosis factor-α (TNFα), and interleukins (IL) -1β, -6, and -10. EVs stained with markers associated with exosomes (CD63), microvesicles (VAMP3), and apoptotic bodies (THSD1) were characterized using imaging flow cytometry and vesicle flow cytometry. The selection event induced significant changes in circulating BDNF (-43.2%), IGF-I (-24.6%), TNFα (+17.7%), and IL-6 (+13.6%) accompanied by increases in intensities of THSD1+ and VAMP3+ EVs (all P < 0.05). Higher concentrations of IL-1β and IL-10 were positively associated with THSD1+ EVs (P < 0.05). Military operational stress altered the EV profile. Surface markers associated with apoptotic bodies were positively correlated with an inflammatory response. Future studies should consider a multiomics assessment of EV cargo to discern canonical pathways that may be mediated by EVs during military stress.
Keywords: apoptotic bodies; biomarkers; exosomes; microvesicles; stress.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Beckner ME, Conkright WR, Eagle SR, Martin BJ, Sinnott AM, LaGoy AD, Proessl F, Lovalekar M, Jabloner LR, Roma PG, Basner M, Ferrarelli F, Germain A, Flanagan SD, Connaboy C, Nindl BC. Impact of simulated military operational stress on executive function relative to trait resilience, aerobic fitness, and neuroendocrine biomarkers. Physiol Behav 236: 113413, 2021. doi:10.1016/j.physbeh.2021.113413. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

