Improving Photodynamic Therapy Anticancer Activity of a Mitochondria-Targeted Coumarin Photosensitizer Using a Polyurethane-Polyurea Hybrid Nanocarrier
- PMID: 35695426
- PMCID: PMC9277592
- DOI: 10.1021/acs.biomac.2c00361
Improving Photodynamic Therapy Anticancer Activity of a Mitochondria-Targeted Coumarin Photosensitizer Using a Polyurethane-Polyurea Hybrid Nanocarrier
Abstract
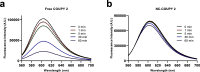
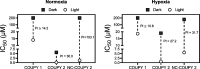
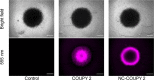
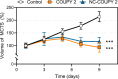
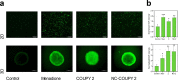
Integration of photosensitizers (PSs) within nanoscale delivery systems offers great potential for overcoming some of the "Achiles' heels" of photodynamic therapy (PDT). Herein, we have encapsulated a mitochondria-targeted coumarin PS into amphoteric polyurethane-polyurea hybrid nanocapsules (NCs) with the aim of developing novel nanoPDT agents. The synthesis of coumarin-loaded NCs involved the nanoemulsification of a suitable prepolymer in the presence of a PS without needing external surfactants, and the resulting small nanoparticles showed improved photostability compared with the free compound. Nanoencapsulation reduced dark cytotoxicity of the coumarin PS and significantly improved in vitro photoactivity with red light toward cancer cells, which resulted in higher phototherapeutic indexes compared to free PS. Importantly, this nanoformulation impaired tumoral growth of clinically relevant three-dimensional multicellular tumor spheroids. Mitochondrial photodamage along with reactive oxygen species (ROS) photogeneration was found to trigger autophagy and apoptotic cell death of cancer cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Zheng Q.; Juette M. F.; Jockusch S.; Wasserman M. R.; Zhou Z.; Altmana R. B.; Blanchard S. C. Ultra-stable organic fluorophores for single-molecule research. Chem. Soc. Rev. 2014, 43, 1044–1056. 10.1039/C3CS60237K. - DOI - PMC - PubMed
- Zheng Q.; Lavis L. D. Development of photostable fluorophores for molecular imaging. Curr. Opin. Chem. Biol. 2017, 39, 32–38. 10.1016/j.cbpa.2017.04.017. - DOI - PubMed
- Freidus L. G.; Pradeep P.; Kumar P.; Choonara Y. E.; Pillay V. Alternative fluorophores designed for advanced molecular imaging. Drug Discovery Today 2018, 23, 115–133. 10.1016/j.drudis.2017.09.008. - DOI - PubMed
- Colas K.; Doloczki S.; Urrutia M. P.; Dyrager C. Prevalent bioimaging scaffolds: Synthesis, photophysical properties and applications. Eur. J. Org. Chem. 2021, 2133–2144. 10.1002/ejoc.202001658. - DOI
-
- Yun S. H.; Kwok S. J. J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 000810.1038/s41551-016-0008. - DOI - PMC - PubMed
- Mari C.; Pierroz V.; Ferrari S.; Gasser G. Combination of Ru(II) complexes and light: new frontiers in cancer therapy. Chem. Sci. 2015, 6, 2660–2686. 10.1039/C4SC03759F. - DOI - PMC - PubMed
- Zhou Z.; Song J.; Nie L.; Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. 10.1039/C6CS00271D. - DOI - PMC - PubMed
-
- Rodriguez-Amigo B.; Planas O.; Bresoli-Obach R.; Torra J.; Ruiz-Gonzalez R.; Nonell S. Photosensitisers for photodynamic therapy: state of the art and perspectives. Compr. Ser. Photochem. Photobiol. Sci. 2016, 15, 25–64. 10.1039/9781782626824-00023. - DOI
- Abrahamse H.; Hamblin M. R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. 10.1042/BJ20150942. - DOI - PMC - PubMed
- Feng G.; Zhang G.-Q.; Ding D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. 10.1039/D0CS00671H. - DOI - PubMed
- Gao Z.; Li C.; Shen J.; Dingy D. Organic optical agents for image-guided combined cancer therapy. Biomed. Mater. 2021, 16, 04200910.1088/1748-605X/abf980. - DOI - PubMed
-
- Ormond A. B.; Freeman H. S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. 10.3390/ma6030817. - DOI - PMC - PubMed
- Zhao X.; Liu J.; Fan J.; Chao H.; Peng Z. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. 10.1039/D0CS00173B. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

