Bacterial Strain-Dependent Dissociation of Cell Recruitment and Cell-to-Cell Spread in Early M. tuberculosis Infection
- PMID: 35695454
- PMCID: PMC9239178
- DOI: 10.1128/mbio.01332-22
Bacterial Strain-Dependent Dissociation of Cell Recruitment and Cell-to-Cell Spread in Early M. tuberculosis Infection
Abstract
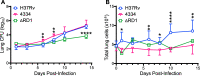
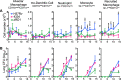
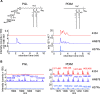
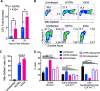
In the initial stage of respiratory infection, Mycobacterium tuberculosis traverses from alveolar macrophages to phenotypically diverse monocyte-derived phagocytes and neutrophils in the lung parenchyma. Here, we compare the in vivo kinetics of early bacterial growth and cell-to-cell spread of two strains of M. tuberculosis: a lineage 2 strain, 4334, and the widely studied lineage 4 strain H37Rv. Using flow cytometry, live cell sorting of phenotypic subsets, and quantitation of bacteria in cells of the distinct subsets, we found that 4334 induces less leukocyte influx into the lungs but demonstrates earlier population expansion and cell-to-cell spread. The earlier spread of 4334 to recruited cells, including monocyte-derived dendritic cells, is accompanied by earlier and greater magnitude of CD4+ T cell activation. The results provide evidence that strain-specific differences in interactions with lung leukocytes can shape adaptive immune responses in vivo. IMPORTANCE Tuberculosis is a leading infectious disease killer worldwide and is caused by Mycobacterium tuberculosis. After exposure to M. tuberculosis, outcomes range from apparent elimination to active disease. Early innate immune responses may contribute to differences in outcomes, yet it is not known how bacterial strains alter the early dynamics of innate immune and T cell responses. We infected mice with distinct strains of M. tuberculosis and discovered striking differences in innate cellular recruitment, cell-to-cell spread of bacteria in the lungs, and kinetics of initiation of antigen-specific CD4 T cell responses. We also found that M. tuberculosis can spread beyond alveolar macrophages even before a large influx of inflammatory cells. These results provide evidence that distinct strains of M. tuberculosis can exhibit differential kinetics in cell-to-cell spread which is not directly linked to early recruitment of phagocytes but is subsequently linked to adaptive immune responses.
Keywords: Beijing strain; Mycobacterium tuberculosis; T cell activation; T cell priming; dendritic cells; innate immunity; innate response; macrophage; strain diversity; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
