Albumin-mediated extracellular zinc speciation drives cellular zinc uptake
- PMID: 35695483
- PMCID: PMC9244874
- DOI: 10.1039/d2cc02278h
Albumin-mediated extracellular zinc speciation drives cellular zinc uptake
Abstract
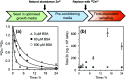
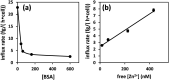
The role of the extracellular medium in influencing metal uptake into cells has not been described quantitatively. In a chemically-defined model system containing albumin, zinc influx into endothelial cells correlates with the extracellular free zinc concentration. Allosteric inhibition of zinc-binding to albumin by free fatty acids increased zinc flux.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Crosstalk between zinc and free fatty acids in plasma.Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Apr;1864(4):532-542. doi: 10.1016/j.bbalip.2018.09.007. Epub 2018 Sep 25. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30266430 Free PMC article. Review.
-
Allosteric modulation of zinc speciation by fatty acids.Biochim Biophys Acta. 2013 Dec;1830(12):5456-64. doi: 10.1016/j.bbagen.2013.05.028. Epub 2013 May 29. Biochim Biophys Acta. 2013. PMID: 23726993 Review.
-
A metalloproteomic analysis of interactions between plasma proteins and zinc: elevated fatty acid levels affect zinc distribution.Metallomics. 2019 Nov 1;11(11):1805-1819. doi: 10.1039/c9mt00177h. Epub 2019 Oct 15. Metallomics. 2019. PMID: 31612889
-
Zinc uptake by human erythrocytes with and without serum albumins in the medium.Biol Trace Elem Res. 2001 Winter;84(1-3):45-56. doi: 10.1385/BTER:84:1-3:045. Biol Trace Elem Res. 2001. PMID: 11817695
-
A molecular mechanism for modulating plasma Zn speciation by fatty acids.J Am Chem Soc. 2012 Jan 25;134(3):1454-7. doi: 10.1021/ja210496n. Epub 2012 Jan 12. J Am Chem Soc. 2012. PMID: 22239162 Free PMC article.
Cited by
-
The role of Zn2+ in shaping intracellular Ca2+ dynamics in the heart.J Gen Physiol. 2023 Jul 3;155(7):e202213206. doi: 10.1085/jgp.202213206. Epub 2023 Jun 16. J Gen Physiol. 2023. PMID: 37326614 Free PMC article. Review.
-
EPR spectroscopic characterisation of native CuII-binding sites in human serum albumin.Dalton Trans. 2024 Aug 13;53(32):13529-13536. doi: 10.1039/d4dt00892h. Dalton Trans. 2024. PMID: 39072685 Free PMC article.
-
Reactive Cu2+-peptide intermediates revealed by kinetic studies gain relevance by matching time windows in copper metallomics.Metallomics. 2023 Feb 16;15(2):mfad007. doi: 10.1093/mtomcs/mfad007. Metallomics. 2023. PMID: 36787891 Free PMC article.
-
Properties of the major Zn2+-binding site of human alpha-fetoprotein, a potential foetal plasma zinc carrier.Chem Commun (Camb). 2025 Jan 28;61(10):2099-2102. doi: 10.1039/d4cc06611a. Chem Commun (Camb). 2025. PMID: 39801276 Free PMC article.
-
Unlocking the brain's zinc code: implications for cognitive function and disease.Front Biophys. 2024;2:1406868. doi: 10.3389/frbis.2024.1406868. Epub 2024 Jun 11. Front Biophys. 2024. PMID: 39758530 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

