Endogenous glutamine is rate-limiting for anti-CD3 and anti-CD28 induced CD4+ T-cell proliferation and glycolytic activity under hypoxia and normoxia
- PMID: 35695514
- PMCID: PMC9246347
- DOI: 10.1042/BCJ20220144
Endogenous glutamine is rate-limiting for anti-CD3 and anti-CD28 induced CD4+ T-cell proliferation and glycolytic activity under hypoxia and normoxia
Abstract
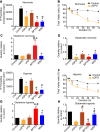
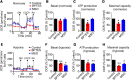
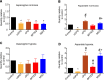
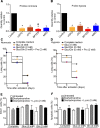
To meet the demand for energy and biomass, T lymphocytes (T cells) activated to proliferation and clonal expansion, require uptake and metabolism of glucose (Gluc) and the amino acid (AA) glutamine (Gln). Whereas exogenous Gln is converted to glutamate (Glu) by glutaminase (GLS), Gln is also synthesized from the endogenous pool of AA through Glu and activity of glutamine synthase (GS). Most of this knowledge comes from studies on cell cultures under ambient oxygen conditions (normoxia, 21% O2). However, in vivo, antigen induced T-cell activation often occurs under moderately hypoxic (1-4% O2) conditions and at various levels of exogenous nutrients. Here, CD4+ T cells were stimulated for 72 h with antibodies targeting the CD3 and CD28 markers at normoxia and hypoxia (1% O2). This was done in the presence and absence of the GLS and GS inhibitors, Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl) ethyl sulfide (BPTES) and methionine sulfoximine (MSO) and at various combinations of exogenous Gluc, Gln and pyruvate (Pyr) for the last 12 h of stimulation. We found that T-cell proliferation, viability and levels of endogenous AA were significantly influenced by the availability of exogenous Gln, Gluc and Pyr as well as inhibition of GLS and GS. Moreover, inhibition of GLS and GS and levels of oxygen differentially influenced oxygen consumption rate (OCR) and extracellular acidification rate (ECAR). Finally, BPTES-dependent down-regulation of ECAR was associated with reduced hexokinase (HK) activity at both normoxia and hypoxia. Our results demonstrate that Gln availability and metabolism is rate-limiting for CD4+ T-cell activity.
Keywords: BPTES; CD4+ T cells; glutamine; hypoxia; metabolism; normoxia.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

