Design, Synthesis and Characterization of a Visible-Light-Sensitive Molecular Switch and Its PEGylation Towards a Self-Assembling Molecule
- PMID: 35695822
- PMCID: PMC9541190
- DOI: 10.1002/chem.202201477
Design, Synthesis and Characterization of a Visible-Light-Sensitive Molecular Switch and Its PEGylation Towards a Self-Assembling Molecule
Abstract
HBDI-like chromophores represent a novel set of biomimetic switches mimicking the fluorophore of the green fluorescent protein that are currently studied with the hope to expand the molecular switch/motor toolbox. However, until now members capable of absorbing visible light in their neutral (i. e. non-anionic) form have not been reported. In this contribution we report the preparation of an HBDI-like chromophore based on a 3-phenylbenzofulvene scaffold capable of absorbing blue light and photoisomerizing on the picosecond timescale. More specifically, we show that double-bond photoisomerization occurs in both the E-to-Z and Z-to-E directions and that these can be controlled by irradiating with blue and UV light, respectively. Finally, as a preliminary applicative result, we report the incorporation of the chromophore in an amphiphilic molecule and demonstrate the formation of a visible-light-sensitive nanoaggregated state in water.
Keywords: HBDI-like chromophores; light-driven molecular switches; light-sensitive molecules; nanoaggregates; photoswitches; self-assembling molecules.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

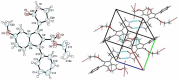


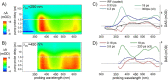






References
-
- Feringa B. L., Koumura N., Van Delden R. A., Ter Wiel M. K. J., Appl. Phys. A 2002, 75, 301–308.
-
- Baroncini M., Silvi S., Credi A., Chem. Rev. 2020, 120, 200–268. - PubMed
-
- AzimiHashemi N., Erbguth K., Vogt A., Riemensperger T., Rauch E., Woodmansee D., Nagpal J., Brauner M., Sheves M., Fiala A., Kattner L., Trauner D., Hegemann P., Gottschalk A., Liewald J. F., Nat. Commun. 2014, 5, 5810. - PubMed
-
- Pagano K., Paolino M., Fusi S., Zanirato V., Trapella C., Giuliani G., Cappelli A., Zanzoni S., Molinari H., Ragona L., Olivucci M., J. Phys. Chem. Lett. 2019, 10, 2235–2243. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

