Acarbose attenuates migration/proliferation via targeting microRNA-143 in vascular smooth muscle cells under diabetic conditions
- PMID: 35696095
- PMCID: PMC9261793
- DOI: 10.38212/2224-6614.1241
Acarbose attenuates migration/proliferation via targeting microRNA-143 in vascular smooth muscle cells under diabetic conditions
Abstract
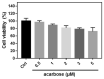
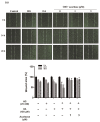
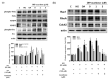
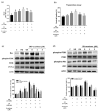
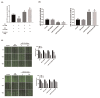
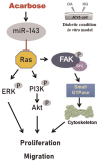
Acarbose (an α-glucosidase inhibitor) has been demonstrated to reduce the progression of atherosclerosis without affecting serum levels of glucose in rabbits fed a high cholesterol diet. The main focus of recent atherosclerosis studies has been microRNA targets. However, the mechanism by which acarbose targets miRNA-mediated atherosclerosis remains unclear. This study aimed to evaluate the effect of acarbose on microRNA-related regulation of rat aortic vascular smooth cell line (A7r5 cell) migration and proliferation induced by diabetic conditions. We reported that acabose exhibit significantly inhibits proliferative and cell migration abilities in A7r5 cells. The expression of protein and levels of mRNA were measured by Western blot analysis and real-time PCR. Acarbose inhibited the phosphorylation of focal adhesion kinase (FAK) and phosphoinositide 3-kinases (PI3K)/protein kinase B (Akt), Ras signals, small GTPase proteins expression to attenuate cell migration and proliferation. Furthermore, acarbose upregulated the expression of miR-143, and transfected miR-143 mimic and its inhibitor to explore its mechanism. In conclusion, acarbose reduces VSMC migration and proliferation via upregulating miR-143 to inhibit Ras-related signaling, and potentially prevention of atherosclerosis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Sacks FM, Lichtenstein AH, Wu JH, Appel LJ, Creager MA, Kris-Etherton PM, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American heart association. Circulation. 2017;136:e1–23. - PubMed
-
- Piché M-E, Poirier P, Lemieux I, Després J-P. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61:103–13. - PubMed
-
- Standl E, Schnell O, McGuire DK, Ceriello A, Ryden L. Integration of recent evidence into management of patients with atherosclerotic cardiovascular disease and type 2 diabetes. Lancet Diab Endocrinol. 2017;5:391–402. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
