Identification of Caveolae-Associated Protein 4 Autoantibodies as a Biomarker of Immune-Mediated Rippling Muscle Disease in Adults
- PMID: 35696196
- PMCID: PMC9361081
- DOI: 10.1001/jamaneurol.2022.1357
Identification of Caveolae-Associated Protein 4 Autoantibodies as a Biomarker of Immune-Mediated Rippling Muscle Disease in Adults
Abstract
Importance: Immune-mediated rippling muscle disease (iRMD) is a rare myopathy characterized by wavelike muscle contractions (rippling) and percussion- or stretch-induced muscle mounding. A serological biomarker of this disease is lacking.
Objective: To describe a novel autoantibody biomarker of iRMD and report associated clinicopathological characteristics.
Design, setting, and participants: This retrospective cohort study evaluated archived sera from 10 adult patients at tertiary care centers at the Mayo Clinic, Rochester, Minnesota, and Brigham & Women's Hospital, Boston, Massachusetts, who were diagnosed with iRMD by neuromuscular specialists in 2000 and 2021, based on the presence of electrically silent percussion- or stretch-induced muscle rippling and percussion-induced rapid muscle contraction with or without muscle mounding and an autoimmune basis. Sera were evaluated for a common biomarker using phage immunoprecipitation sequencing. Myopathology consistent with iRMD was documented in most patients. The median (range) follow-up was 18 (1-30) months.
Exposures: Diagnosis of iRMD.
Main outcomes and measures: Detection of a common autoantibody in serum of patients sharing similar clinical and myopathological features.
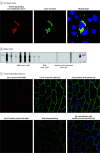
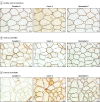
Results: Seven male individuals and 3 female individuals with iRMD were identified (median [range] age at onset, 60 [18-76] years). An IgG autoantibody specific for caveolae-associated protein 4 (cavin-4) was identified in serum of patients with iRMD using human proteome phage immunoprecipitation sequencing. Immunoassays using recombinant cavin-4 confirmed cavin-4 IgG seropositivity in 8 of 10 patients with iRMD. Results for healthy and disease-control individuals (n = 241, including myasthenia gravis and immune-mediated myopathies) were cavin-4 IgG seronegative. Six of the 8 individuals with cavin-4 IgG were male, and the median (range) age was 60 (18-76) years. Initial symptoms included rippling of lower limb muscles in 5 of 8 individuals or all limb muscles in 2 of 8 sparing bulbar muscles, fatigue in 9 of 10, mild proximal weakness in 3 of 8, and isolated myalgia in 1 of 8, followed by development of diffuse rippling. All patients had percussion-induced muscle rippling and half had percussion- or stretch-induced muscle mounding. Four of the 10 patients had proximal weakness. Plasma creatine kinase was elevated in all but 1 patient. Six of the 10 patients underwent malignancy screening; cancer was detected prospectively in only 1. Muscle biopsy was performed in 7 of the 8 patients with cavin-4 IgG; 6 of 6 specimens analyzed immunohistochemically revealed a mosaic pattern of sarcolemmal cavin-4 immunoreactivity. Three of 6 patients whose results were seropositive and who received immunotherapy had complete resolution of symptoms, 1 had mild improvement, and 2 had no change.
Conclusions and relevance: The findings indicate that cavin-4 IgG may be the first specific serological autoantibody biomarker identified in iRMD. Depletion of cavin-4 expression in muscle biopsies of patients with iRMD suggests the potential role of this autoantigen in disease pathogenesis.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

