Updated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Estimates Based on Blood Donations, July 2020-December 2021
- PMID: 35696249
- PMCID: PMC9194752
- DOI: 10.1001/jama.2022.9745
Updated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Estimates Based on Blood Donations, July 2020-December 2021
Abstract
This cross-sectional study examines monthly blood donations from individuals aged 16 years and older to estimate the population with antibodies to SARS-CoV-2 from infection or vaccination.
Conflict of interest statement
Figures
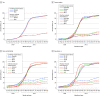
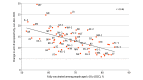
References
-
- Centers for Disease Control and Prevention . COVID data tracker. Accessed March 23, 2022. https://covid.cdc.gov/covid-data-tracker
-
- Ferdinands JM, Rao S, Dixon BE, et al. . Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255-263. doi:10.15585/mmwr.mm7107e2 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

