NADPH-oxidase 4 gene over-expression in peripheral blood lymphocytes of the schizophrenia patients
- PMID: 35696356
- PMCID: PMC9191697
- DOI: 10.1371/journal.pone.0269130
NADPH-oxidase 4 gene over-expression in peripheral blood lymphocytes of the schizophrenia patients
Abstract
Introduction: Increased systemic oxidative stress is common in schizophrenia (SZ) patients. NADPH-oxidase 4 (NOX4) is the cell oxidoreductase, catalyzing the hydrogen peroxide formation. Presumably, NOX4 is the main oxidative stress factor in a number of diseases such as cardiovascular diseases and cancer. We hypothesized that NOX4 may be involved in the oxidative stress development caused by the disease in the schizophrenic patients' peripheral blood lymphocytes (PBL).
Materials and methods: The SZ group included 100 patients (68 men and 32 women aged 28 ± 11 years). The control group included 60 volunteers (35 men and 25 women aged 25 ± 12 years). Flow cytometry analysis (FCA) was used for DNA damage markers (8-oxodG, ɣH2AX), pro- and antiapoptotic proteins (BAX1 and BCL2) and the master-regulator of anti-oxidant response NRF2 detection in the lymphocytes of the untreated SZ patients (N = 100) and the healthy control (HC, N = 60). FCA and RT-qPCR were used for NOX4 and RNANOX4 detection in the lymphocytes. RT-qPCR was used for mtDNA quantitation in peripheral blood mononuclear cells. Cell-free DNA concentration was determined in blood plasma fluorimetrically.
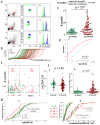
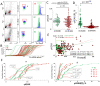
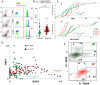
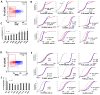
Results: 8-oxodG, NOX4, and BCL2 levels in the PBL in the SZ group were higher than those in the HC group (p < 0.001). ɣH2AX protein level was increased in the subgroup with high 8-oxodG (p<0.02) levels and decreased in the subgroup with low 8-oxodG (p <0.0001) levels. A positive correlation was found between 8-oxodG, ɣH2AX and BAX1 levels in the SZ group (p <10-6). NOX4 level in lymphocytes did not depend on the DNA damage markers values and BAX1 and BCL2 proteins levels. In 15% of PBL of the HC group a small cellular subfraction was found (5-12% of the total lymphocyte pool) with high DNA damage level and elevated BAX1 protein level. The number of such cells was maximal in PBL samples with low NOX4 protein levels.
Conclusion: Significant NOX4 gene expression was found a in SZ patients' lymphocytes, but the corresponding protein is probably not a cause of the DNA damage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Nishioka N., Arnold S.E. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry 2004, 12(2), 167–75. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

