Krüppel-like factor 15 integrated autophagy and gluconeogenesis to maintain glucose homeostasis under 20-hydroxyecdysone regulation
- PMID: 35696369
- PMCID: PMC9191741
- DOI: 10.1371/journal.pgen.1010229
Krüppel-like factor 15 integrated autophagy and gluconeogenesis to maintain glucose homeostasis under 20-hydroxyecdysone regulation
Abstract
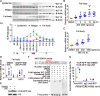
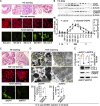
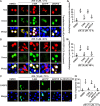
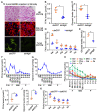
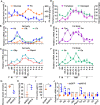
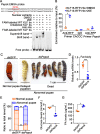
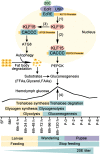
The regulation of glycometabolism homeostasis is vital to maintain health and development of animal and humans; however, the molecular mechanisms by which organisms regulate the glucose metabolism homeostasis from a feeding state switching to a non-feeding state are not fully understood. Using the holometabolous lepidopteran insect Helicoverpa armigera, cotton bollworm, as a model, we revealed that the steroid hormone 20-hydroxyecdysone (20E) upregulated the expression of transcription factor Krüppel-like factor (identified as Klf15) to promote macroautophagy/autophagy, apoptosis and gluconeogenesis during metamorphosis. 20E via its nuclear receptor EcR upregulated Klf15 transcription in the fat body during metamorphosis. Knockdown of Klf15 using RNA interference delayed pupation and repressed autophagy and apoptosis of larval fat body during metamorphosis. KLF15 promoted autophagic flux and transiting to apoptosis. KLF15 bound to the KLF binding site (KLF bs) in the promoter of Atg8 (autophagy-related gene 8/LC3) to upregulate Atg8 expression. Knockdown Atg8 reduced free fatty acids (FFAs), glycerol, free amino acids (FAAs) and glucose levels. However, knockdown of Klf15 accumulated FFAs, glycerol, and FAAs. Glycolysis was switched to gluconeogenesis, trehalose and glycogen synthesis were changed to degradation during metamorphosis, which were accompanied by the variation of the related genes expression. KLF15 upregulated phosphoenolpyruvate carboxykinase (Pepck) expression by binding to KLF bs in the Pepck promoter for gluconeogenesis, which utilised FFAs, glycerol, and FAAs directly or indirectly to increase glucose in the hemolymph. Taken together, 20E via KLF15 integrated autophagy and gluconeogenesis by promoting autophagy-related and gluconeogenesis-related genes expression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

