Seroprevalence of immunoglobulin G antibodies against SARS-CoV-2 in Cyprus
- PMID: 35696396
- PMCID: PMC9191710
- DOI: 10.1371/journal.pone.0269885
Seroprevalence of immunoglobulin G antibodies against SARS-CoV-2 in Cyprus
Abstract
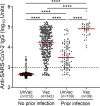
Monitoring the levels of IgG antibodies against the SARS-CoV-2 is important during the coronavirus disease 2019 (COVID-19) pandemic, to plan an adequate and evidence-based public health response. After this study we report that the plasma levels of IgG antibodies against SARS-CoV-2 spike protein were higher in individuals with evidence of prior infection who received at least one dose of either an mRNA-based vaccine (Comirnaty BNT162b2/Pfizer-BioNTech or Spikevax mRNA-1273/Moderna) or an adenoviral-based vaccine (Vaxzervia ChAdOx1 nCoV-19 /Oxford-Astra Zeneca) (n = 39) compared to i) unvaccinated individuals with evidence of prior infection with SARS-CoV-2 (n = 109) and ii) individuals without evidence of prior infection with SARS-CoV-2 who received one or two doses of one of the aforementioned vaccines (n = 342). Our analysis also revealed that regardless of the vaccine technology (mRNA-based and adenoviral vector-based) two doses achieved high anti- SARS-CoV-2 IgG responses. Our results indicate that vaccine-induced responses lead to higher levels of IgG antibodies compared to those produced following infection with the virus. Additionally, in agreement with previous studies, our results suggest that among individuals previously infected with SARS-CoV-2, even a single dose of a vaccine is adequate to elicit high levels of antibody response.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

