The gain of function SCN1A disorder spectrum: novel epilepsy phenotypes and therapeutic implications
- PMID: 35696452
- PMCID: PMC9679167
- DOI: 10.1093/brain/awac210
The gain of function SCN1A disorder spectrum: novel epilepsy phenotypes and therapeutic implications
Abstract
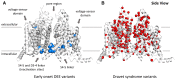
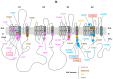
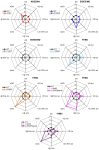
Brain voltage-gated sodium channel NaV1.1 (SCN1A) loss-of-function variants cause the severe epilepsy Dravet syndrome, as well as milder phenotypes associated with genetic epilepsy with febrile seizures plus. Gain of function SCN1A variants are associated with familial hemiplegic migraine type 3. Novel SCN1A-related phenotypes have been described including early infantile developmental and epileptic encephalopathy with movement disorder, and more recently neonatal presentations with arthrogryposis. Here we describe the clinical, genetic and functional evaluation of affected individuals. Thirty-five patients were ascertained via an international collaborative network using a structured clinical questionnaire and from the literature. We performed whole-cell voltage-clamp electrophysiological recordings comparing sodium channels containing wild-type versus variant NaV1.1 subunits. Findings were related to Dravet syndrome and familial hemiplegic migraine type 3 variants. We identified three distinct clinical presentations differing by age at onset and presence of arthrogryposis and/or movement disorder. The most severely affected infants (n = 13) presented with congenital arthrogryposis, neonatal onset epilepsy in the first 3 days of life, tonic seizures and apnoeas, accompanied by a significant movement disorder and profound intellectual disability. Twenty-one patients presented later, between 2 weeks and 3 months of age, with a severe early infantile developmental and epileptic encephalopathy and a movement disorder. One patient presented after 3 months with developmental and epileptic encephalopathy only. Associated SCN1A variants cluster in regions of channel inactivation associated with gain of function, different to Dravet syndrome variants (odds ratio = 17.8; confidence interval = 5.4-69.3; P = 1.3 × 10-7). Functional studies of both epilepsy and familial hemiplegic migraine type 3 variants reveal alterations of gating properties in keeping with neuronal hyperexcitability. While epilepsy variants result in a moderate increase in action current amplitude consistent with mild gain of function, familial hemiplegic migraine type 3 variants induce a larger effect on gating properties, in particular the increase of persistent current, resulting in a large increase of action current amplitude, consistent with stronger gain of function. Clinically, 13 out of 16 (81%) gain of function variants were associated with a reduction in seizures in response to sodium channel blocker treatment (carbamazepine, oxcarbazepine, phenytoin, lamotrigine or lacosamide) without evidence of symptom exacerbation. Our study expands the spectrum of gain of function SCN1A-related epilepsy phenotypes, defines key clinical features, provides novel insights into the underlying disease mechanisms between SCN1A-related epilepsy and familial hemiplegic migraine type 3, and identifies sodium channel blockers as potentially efficacious therapies. Gain of function disease should be considered in early onset epilepsies with a pathogenic SCN1A variant and non-Dravet syndrome phenotype.
Keywords: SCN1A; arthrogryposis; epilepsy; gain of function; movement disorder.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures





References
-
- Wolff M, Johannesen KM, Hedrich UBS, et al. . Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain. 2017;140:1316–1336. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

