Essential functions of mosquito ecdysone importers in development and reproduction
- PMID: 35696563
- PMCID: PMC9231622
- DOI: 10.1073/pnas.2202932119
Essential functions of mosquito ecdysone importers in development and reproduction
Abstract
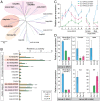
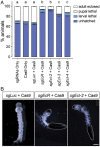
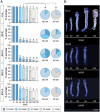
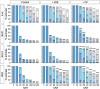
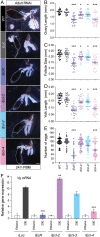
The primary insect steroid hormone ecdysone requires a membrane transporter to enter its target cells. Although an organic anion-transporting polypeptide (OATP) named Ecdysone Importer (EcI) serves this role in the fruit fly Drosophila melanogaster and most likely in other arthropod species, this highly conserved transporter is apparently missing in mosquitoes. Here we report three additional OATPs that facilitate cellular incorporation of ecdysone in Drosophila and the yellow fever mosquito Aedes aegypti. These additional ecdysone importers (EcI-2, -3, and -4) are dispensable for development and reproduction in Drosophila, consistent with the predominant role of EcI. In contrast, in Aedes, EcI-2 is indispensable for ecdysone-mediated development, whereas EcI-4 is critical for vitellogenesis induced by ecdysone in adult females. Altogether, our results indicate unique and essential functions of these additional ecdysone importers in mosquito development and reproduction, making them attractive molecular targets for species- and stage-specific control of ecdysone signaling in mosquitoes.
Keywords: Aedes aegypti; Drosophila melanogaster; ecdysone; organic anion-transporting polypeptide (OATP); vitellogenesis.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Yamanaka N., Ecdysteroid signalling in insects—From biosynthesis to gene expression regulation. Adv. Insect Physiol. 60, 1–36 (2021).
-
- Thomas H. E., Stunnenberg H. G., Stewart A. F., Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and ultraspiracle. Nature 362, 471–475 (1993). - PubMed
-
- Yao T.-P., Segraves W. A., Oro A. E., McKeown M., Evans R. M., Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell 71, 63–72 (1992). - PubMed
-
- Yao T.-P., et al. , Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature 366, 476–479 (1993). - PubMed
-
- Riddiford L. M., Cherbas P., Truman J. W., Ecdysone receptors and their biological actions. Vitam. Horm. 60, 1–73 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

