Radioresistant cells initiate lymphocyte-dependent lung inflammation and IFNγ-dependent mortality in STING gain-of-function mice
- PMID: 35696583
- PMCID: PMC9231608
- DOI: 10.1073/pnas.2202327119
Radioresistant cells initiate lymphocyte-dependent lung inflammation and IFNγ-dependent mortality in STING gain-of-function mice
Abstract
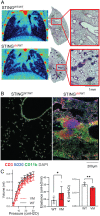
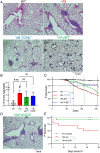
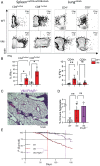
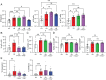
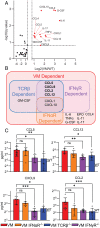
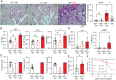
Pediatric patients with constitutively active mutations in the cytosolic double-stranded-DNA-sensing adaptor STING develop an autoinflammatory syndrome known as STING-associated vasculopathy with onset in infancy (SAVI). SAVI patients have elevated interferon-stimulated gene expression and suffer from interstitial lung disease (ILD) with lymphocyte predominate bronchus-associated lymphoid tissue (BALT). Mice harboring SAVI mutations (STING V154M [VM]) that recapitulate human disease also develop lymphocyte-rich BALT. Ablation of either T or B lymphocytes prolongs the survival of SAVI mice, but lung immune aggregates persist, indicating that T cells and B cells can independently be recruited as BALT. VM T cells produced IFNγ, and IFNγR deficiency prolonged the survival of SAVI mice; however, T-cell-dependent recruitment of infiltrating myeloid cells to the lung was IFNγ independent. Lethally irradiated VM recipients fully reconstituted with wild type bone-marrow-derived cells still developed ILD, pointing to a critical role for VM-expressing radioresistant parenchymal and/or stromal cells in the recruitment and activation of pathogenic lymphocytes. We identified lung endothelial cells as radioresistant cells that express STING. Transcriptional analysis of VM endothelial cells revealed up-regulation of chemokines, proinflammatory cytokines, and genes associated with antigen presentation. Together, our data show that VM-expressing radioresistant cells play a key role in the initiation of lung disease in VM mice and provide insights for the treatment of SAVI patients, with implications for ILD associated with other connective tissue disorders.
Keywords: SAVI; STING; endothelial cells; interferon gamma; interstitial lung disease.
Conflict of interest statement
Competing interest statement: The authors declare a competing interest. K.A.F. serves as a scientific advisory board member to Janssen, Moderna, Related Sciences, Generation Bio, and NodThera Inc as well as a consultant for Casma Therapeutics, Jnana Therapeutics, Elicio Therapeutics, and OMass Therapeutics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

