Empirical transmit field bias correction of T1w/T2w myelin maps
- PMID: 35697132
- PMCID: PMC9483036
- DOI: 10.1016/j.neuroimage.2022.119360
Empirical transmit field bias correction of T1w/T2w myelin maps
Abstract
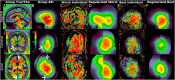
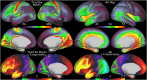
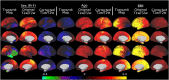
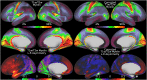
T1-weighted divided by T2-weighted (T1w/T2w) myelin maps were initially developed for neuroanatomical analyses such as identifying cortical areas, but they are increasingly used in statistical comparisons across individuals and groups with other variables of interest. Existing T1w/T2w myelin maps contain radiofrequency transmit field (B1+) biases, which may be correlated with these variables of interest, leading to potentially spurious results. Here we propose two empirical methods for correcting these transmit field biases using either explicit measures of the transmit field or alternatively a 'pseudo-transmit' approach that is highly correlated with the transmit field at 3T. We find that the resulting corrected T1w/T2w myelin maps are both better neuroanatomical measures (e.g., for use in cross-species comparisons), and more appropriate for statistical comparisons of relative T1w/T2w differences across individuals and groups (e.g., sex, age, or body-mass-index) within a consistently acquired study at 3T. We recommend that investigators who use the T1w/T2w approach for mapping cortical myelin use these B1+ transmit field corrected myelin maps going forward.
Copyright © 2022. Published by Elsevier Inc.
Figures






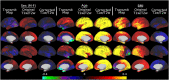
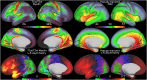

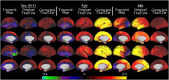
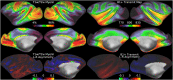

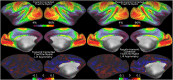

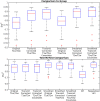
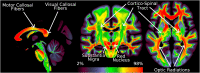
References
-
- Bock N.A., Hashim E., Janik R., Konyer N.B., Weiss M., Stanisz G.J., Turner R., Geyer S. Optimizing T1-weighted imaging of cortical myelin content at 3.0T. Neuroimage. 2013;65:1–12. - PubMed
-
- Bonny J.M., Foucat L., Laurent W., Renou J.P. Optimization of signal intensity andt1-dependent contrast with nonstandard flip angles in spin-echo and inversion-recovery mr imaging. J. Magn. Reson. 1998;130(1):51–57. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

