Sexual violence history predicts changes in vaginal immune parameters during sexual arousal
- PMID: 35697156
- PMCID: PMC9734281
- DOI: 10.1016/j.bbi.2022.06.005
Sexual violence history predicts changes in vaginal immune parameters during sexual arousal
Abstract
Objective: To examine the influence of sexual arousal on vaginal mucosal inflammatory cytokine and antibody production in healthy women with and without histories of childhood and/or adult sexual violence.
Methods: Ninety-one premenopausal healthy women (ages 18-42) attended a single laboratory session in which they provided vaginal fluid samples before and after viewing one neutral and one erotic film. While viewing the films, participants' vaginal sexual arousal was recorded using vaginal photoplethysmography.
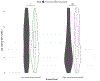
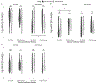
Results: Of the 91 participants, 41 (45%) reported no history of sexual violence, 17 (19%) reported a history of childhood sexual abuse (CSA) only, 19 (21%) reported a history of adult sexual assault (ASA) only, and 10 (11%) reported a history of both CSA and ASA, with 4 participants choosing not to provide information on their sexual violence history. For women with a history of ASA but not CSA, there was a significant increase in vaginal IL-1β following arousal, while for women with a history of CSA (with or without ASA), there was a significant decrease. Women without CSA histories had a significant increase in vaginal IgA following sexual arousal, while women with CSA histories had a decrease.
Conclusion: Sexual arousal possibly plays a role in modifying vaginal immune responses in young, healthy women. Moreover, these effects may vary depending upon sexual assault histories, such that relative to women without assault histories, women with a history of early life sexual trauma showed significantly altered vaginal immune responses following sexual arousal. If replicated, these findings may help explain the increased risk for sexually transmitted infections observed among women with sexual assault histories.
Keywords: Antibody; Childhood sexual abuse; Cytokine; Sexual arousal; Sexual violence; Vaginal; Women.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Apalata T, Longo-Mbenza B, Prasad S, 2020. The role of T helper 17 (Th17) and regulatory T cells (Treg) in the pathogenesis of vulvovaginal candidiasis among HIV-infected women. Int. J. Microbiol 2020, 1–10.
-
- Archary D, Liebenberg LJ, Werner L, Tulsi S, Majola N, Naicker N, Dlamini S, Hope TJ, Samsunder N, Abdool Karim SS, Morris L, Passmore JA, Garrett NJ, Tachedjian G, 2015. Randomized cross-sectional study to compare HIV-1 specific antibody and cytokine concentrations in female genital secretions obtained by menstrual cup and cervicovaginal lavage. PLoS ONE 10 (7), e0131906. - PMC - PubMed
-
- Bates D, Mächler M, Bolker B, & Walker S. (2014). Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

