Change in covid-19 risk over time following vaccination with CoronaVac: test negative case-control study
- PMID: 35697361
- PMCID: PMC9189440
- DOI: 10.1136/bmj-2022-070102
Change in covid-19 risk over time following vaccination with CoronaVac: test negative case-control study
Abstract
Objective: To estimate the change in odds of covid-19 over time following primary series completion of the inactivated whole virus vaccine CoronaVac (Sinovac Biotech) in São Paulo State, Brazil.
Design: Test negative case-control study.
Setting: Community testing for covid-19 in São Paulo State, Brazil.
Participants: Adults aged ≥18 years who were residents of São Paulo state, had received two doses of CoronaVac, did not have a laboratory confirmed SARS-CoV-2 infection before vaccination, and underwent reverse transcription polymerase chain reaction (RT-PCR) testing for SARS-CoV-2 from 17 January to 14 December 2021. Cases were matched to test negative controls by age (in 5 year bands), municipality of residence, healthcare worker status, and epidemiological week of RT-PCR test.
Main outcome measures: RT-PCR confirmed symptomatic covid-19 and associated hospital admissions and deaths. Conditional logistic regression was adjusted for sex, number of covid-19 associated comorbidities, race, and previous acute respiratory illness.
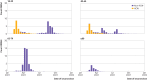
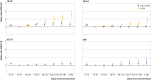
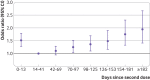
Results: From 202 741 eligible people, 52 170 cases with symptomatic covid-19 and 69 115 test negative controls with covid-19 symptoms were formed into 43 257 matched sets. Adjusted odds ratios of symptomatic covid-19 increased with time since completion of the vaccination series. The increase in odds was greater in younger people and among healthcare workers, although sensitivity analyses suggested that this was in part due to bias. In addition, the adjusted odds ratios of covid-19 related hospital admission or death significantly increased with time compared with the odds 14-41 days after series completion: from 1.25 (95% confidence interval 1.04 to 1.51) at 70-97 days up to 1.94 (1.41 to 2.67) from 182 days onwards.
Conclusions: Significant increases in the risk of moderate and severe covid-19 outcomes occurred three months after primary vaccination with CoronaVac among people aged 65 and older. These findings provide supportive evidence for the implementation of vaccine boosters in these populations who received this inactivated vaccine. Studies of waning should include analyses designed to uncover common biases.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Institute for Allergy and Infectious Diseases and from the Sendas Family and Beatrice Kleinberg Neuwirth Funds; MDTH and DATC report a contract from Merck (to the University of Florida) for research unrelated to this manuscript; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Update of
-
Change in COVID-19 risk over time following vaccination with CoronaVac: A testnegative case-control study.medRxiv [Preprint]. 2021 Dec 24:2021.12.23.21268335. doi: 10.1101/2021.12.23.21268335. medRxiv. 2021. Update in: BMJ. 2022 Jun 13;377:e070102. doi: 10.1136/bmj-2022-070102. PMID: 34988559 Free PMC article. Updated. Preprint.
References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/.
-
- Mallapaty S. China’s COVID vaccines have been crucial — now immunity is waning. 2021. https://www.nature.com/articles/d41586-021-02796-w. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
