Development and validation of a decision support tool for the diagnosis of acute heart failure: systematic review, meta-analysis, and modelling study
- PMID: 35697365
- PMCID: PMC9189738
- DOI: 10.1136/bmj-2021-068424
Development and validation of a decision support tool for the diagnosis of acute heart failure: systematic review, meta-analysis, and modelling study
Abstract
Objectives: To evaluate the diagnostic performance of N-terminal pro-B-type natriuretic peptide (NT-proBNP) thresholds for acute heart failure and to develop and validate a decision support tool that combines NT-proBNP concentrations with clinical characteristics.
Design: Individual patient level data meta-analysis and modelling study.
Setting: Fourteen studies from 13 countries, including randomised controlled trials and prospective observational studies.
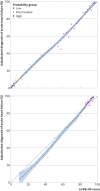
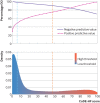
Participants: Individual patient level data for 10 369 patients with suspected acute heart failure were pooled for the meta-analysis to evaluate NT-proBNP thresholds. A decision support tool (Collaboration for the Diagnosis and Evaluation of Heart Failure (CoDE-HF)) that combines NT-proBNP with clinical variables to report the probability of acute heart failure for an individual patient was developed and validated.
Main outcome measure: Adjudicated diagnosis of acute heart failure.
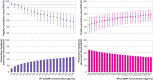
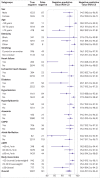
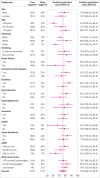
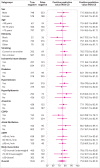
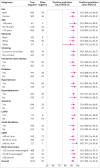
Results: Overall, 43.9% (4549/10 369) of patients had an adjudicated diagnosis of acute heart failure (73.3% (2286/3119) and 29.0% (1802/6208) in those with and without previous heart failure, respectively). The negative predictive value of the guideline recommended rule-out threshold of 300 pg/mL was 94.6% (95% confidence interval 91.9% to 96.4%); despite use of age specific rule-in thresholds, the positive predictive value varied at 61.0% (55.3% to 66.4%), 73.5% (62.3% to 82.3%), and 80.2% (70.9% to 87.1%), in patients aged <50 years, 50-75 years, and >75 years, respectively. Performance varied in most subgroups, particularly patients with obesity, renal impairment, or previous heart failure. CoDE-HF was well calibrated, with excellent discrimination in patients with and without previous heart failure (area under the receiver operator curve 0.846 (0.830 to 0.862) and 0.925 (0.919 to 0.932) and Brier scores of 0.130 and 0.099, respectively). In patients without previous heart failure, the diagnostic performance was consistent across all subgroups, with 40.3% (2502/6208) identified at low probability (negative predictive value of 98.6%, 97.8% to 99.1%) and 28.0% (1737/6208) at high probability (positive predictive value of 75.0%, 65.7% to 82.5%) of having acute heart failure.
Conclusions: In an international, collaborative evaluation of the diagnostic performance of NT-proBNP, guideline recommended thresholds to diagnose acute heart failure varied substantially in important patient subgroups. The CoDE-HF decision support tool incorporating NT-proBNP as a continuous measure and other clinical variables provides a more consistent, accurate, and individualised approach.
Study registration: PROSPERO CRD42019159407.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the British Heart Foundation, Medical Research Council, and Chief Scientist Office; Y-EC has received honorariums for lectures and presentations from Biomérieux, Roche Diagnostics, and Thermo Fisher; CdF has received consulting fees from Fuji Rebio, Ortho Diagnostics, Quidel, and Roche Diagnostics and a patent entitled “Methods for assessing differential risk for developing heart failure” (patent number: PCT/US2015/029838); SS has received a grant from Roche Diagnostics and a patent entitled “Methods for assessing differential risk for developing heart failure” (patent number: PCT/US2015/029838); ABG has received personal fees and non-financial support from Roche Diagnostics during the conduct of the study and personal fees from Abbott and AstraZeneca, grants, personal fees, and non-financial support from Boehringer-Ingelheim, and personal fees and non-financial support from Novartis and Vifor outside the submitted work. YP has received consulting fees from Roche Diagnostics, Pfizer, and Forbion and honorariums from CVOI and Daiichi Sankyo; HKG has received grants from Roche Diagnostics, Jana Care, Ortho Clinical, Novartis, Pfizer, Alnylam, and Akcea (IONIS), consulting fees from Amgen, Eko, Merck, and Pfizer, and stock in Eko; JCW works as a biostatistician at the biotech company BRAHMS GmbH, part of Thermo Fisher Scientific; MM has received grants from Health Care Research Projects and Biomarker Research and personal fees from Consulting outside the submitted work; ASVS has received speaker fees from Abbott Diagnostics outside the submitted work; AMR has received grants, personal fees, and non-financial support from Roche Diagnostics outside the submitted work; JJVM has received consulting fees from Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Dal-Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos, payments for advisory boards, symposiums, or lectures from Abbott, Alkem Metabolics, Canadian Medical and Surgical Knowledge Translation Research Group, Eris Lifesciences, Hikma, Lupin, Sun Pharmaceuticals, Medscape/Heart.Org, ProAdWise Communications, Radcliffe Cardiology, Servier, and the Corpus, has participated on a data safety monitoring board or advisory board for Cardialysis (MONITOR study) and Merck (VICTORIA trial), and works as company director for Global Clinical Trial Partners Ltd (GCTP) outside the submitted work; CM has received grants and non-financial support from several diagnostic companies during the conduct of the study and grants, personal fees, and non-financial support from several diagnostic companies outside the submitted work; JJ has received grants from Abbott Diagnostics, Innolife, and Novartis and consulting fees from Abbott, Jana Care, Novartis, Roche Diagnostics, Bristol-Myers Squibb, Janssen, and Prevencio; NLM has received grants from Siemens Healthineers, consulting fees from Roche Diagnostics and LumiraDx, and speaker fees from Abbott Diagnostics and Siemens Healthineers outside the submitted work; KKL, DD, and NLM are employed by the University of Edinburgh, which has filed a patent on the CoDE-HF score (patent reference: PCT/GB2021/051470); no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- National Institute for Cardiovascular Outcomes Research. National Heart Failure Audit 2019 Summary Report. 2019. https://www.nicor.org.uk/wp-content/uploads/2019/09/Heart-Failure-2019-R....
-
- Roberts E, Ludman AJ, Dworzynski K, et al. NICE Guideline Development Group for Acute Heart Failure . The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ 2015;350:h910. 10.1136/bmj.h910 - DOI - PMC - PubMed
-
- National Institute for Health and Care Excellence. Acute heart failure: diagnosis and management. Clinical Guideline 187. 2014. https://www.nice.org.uk/guidance/cg187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
