The cation exchanger Letm1, circadian rhythms, and NAD(H) levels interconnect in diurnal zebrafish
- PMID: 35697381
- PMCID: PMC9191620
- DOI: 10.26508/lsa.202101194
The cation exchanger Letm1, circadian rhythms, and NAD(H) levels interconnect in diurnal zebrafish
Abstract
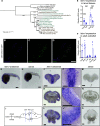
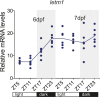
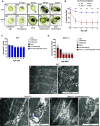
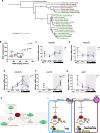
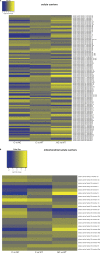
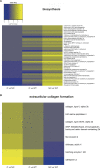
Mitochondria are fundamental for life and require balanced ion exchange to maintain proper functioning. The mitochondrial cation exchanger LETM1 sparks interest because of its pathophysiological role in seizures in the Wolf Hirschhorn Syndrome (WHS). Despite observation of sleep disorganization in epileptic WHS patients, and growing studies linking mitochondria and epilepsy to circadian rhythms, LETM1 has not been studied from the chronobiological perspective. Here we established a viable letm1 knock-out, using the diurnal vertebrate Danio rerio to study the metabolic and chronobiological consequences of letm1 deficiency. We report diurnal rhythms of Letm1 protein levels in wild-type fish. We show that mitochondrial nucleotide metabolism is deregulated in letm1-/- mutant fish, the rate-limiting enzyme of NAD+ production is up-regulated, while NAD+ and NADH pools are reduced. These changes were associated with increased expression amplitude of circadian core clock genes in letm1-/- compared with wild-type under light/dark conditions, suggesting decreased NAD(H) levels as a possible mechanism for circadian system perturbation in Letm1 deficiency. Replenishing NAD pool may ameliorate WHS-associated sleep and neurological disorders.
© 2022 Dao et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
