Neuropathology and emerging biomarkers in corticobasal syndrome
- PMID: 35697501
- PMCID: PMC9380481
- DOI: 10.1136/jnnp-2021-328586
Neuropathology and emerging biomarkers in corticobasal syndrome
Abstract
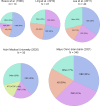
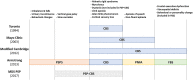
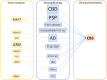
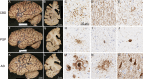
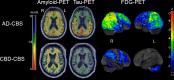
Corticobasal syndrome (CBS) is a clinical syndrome characterised by progressive asymmetric limb rigidity and apraxia with dystonia, myoclonus, cortical sensory loss and alien limb phenomenon. Corticobasal degeneration (CBD) is one of the most common underlying pathologies of CBS, but other disorders, such as progressive supranuclear palsy (PSP), Alzheimer's disease (AD) and frontotemporal lobar degeneration with TDP-43 inclusions, are also associated with this syndrome.In this review, we describe common and rare neuropathological findings in CBS, including tauopathies, synucleinopathies, TDP-43 proteinopathies, fused in sarcoma proteinopathy, prion disease (Creutzfeldt-Jakob disease) and cerebrovascular disease, based on a narrative review of the literature and clinicopathological studies from two brain banks. Genetic mutations associated with CBS, including GRN and MAPT, are also reviewed. Clinicopathological studies on neurodegenerative disorders associated with CBS have shown that regardless of the underlying pathology, frontoparietal, as well as motor and premotor pathology is associated with CBS. Clinical features that can predict the underlying pathology of CBS remain unclear. Using AD-related biomarkers (ie, amyloid and tau positron emission tomography (PET) and fluid biomarkers), CBS caused by AD often can be differentiated from other causes of CBS. Tau PET may help distinguish AD from other tauopathies and non-tauopathies, but it remains challenging to differentiate non-AD tauopathies, especially PSP and CBD. Although the current clinical diagnostic criteria for CBS have suboptimal sensitivity and specificity, emerging biomarkers hold promise for future improvements in the diagnosis of underlying pathology in patients with CBS.
Keywords: alzheimer's disease; corticobasal degeneration; genetics; neuropathology; supranuclear palsy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
CBD diagnostic criteria: exclusions as important as inclusions.J Neurol Neurosurg Psychiatry. 2023 Apr;94(4):328. doi: 10.1136/jnnp-2022-330564. Epub 2022 Nov 2. J Neurol Neurosurg Psychiatry. 2023. PMID: 36323508 No abstract available.
References
-
- Lang A, Riley DE, Bergeron C. Cortical-Basal ganglionic degeneration. neurodegenerative diseases. Philadelphia: WB Saunders, 1994: 877–94.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
