Induced humoral immunity of different types of vaccines against most common variants of SARS-CoV-2 in Egypt prior to Omicron outbreak
- PMID: 35697574
- PMCID: PMC9187864
- DOI: 10.1016/j.vaccine.2022.05.086
Induced humoral immunity of different types of vaccines against most common variants of SARS-CoV-2 in Egypt prior to Omicron outbreak
Abstract
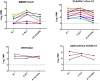
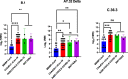
The diversity of SARS-CoV-2 continues to lead to the emergence of new SARS-CoV-2 variants. SARS-CoV-2 antibody assays are crucial in managing the COVID-19 pandemic by determining the neutralizing antibody response. This study aims to investigate vaccine-induced antibodies against most common variants of SARS-CoV-2 in Egypt. Sera samples were collected from vaccinated participants and neutralizing activity against the SARS-CoV-2 variants was determined using microneutralization assay. Our results show that the BNT162b2 (Pfizer-BioNTech), ChAdOx1 nCov-19 (AstraZeneca), and Ad26.COV2.S COVID-19 (Janssen) vaccines elicited neutralizing antibody responses more than the BBIBP-CorV vaccine (Sinopharm) against B.1, C.36.3, and AY.32 (Delta) variants. While vaccines remain highly effective in managing the COVID-19 pandemic, ongoing monitoring of vaccine effectiveness is needed.
Keywords: COVID-19 pandemic; Microneutralization assay; Neutralizing antibodies; SARS-CoV-2; SARS-CoV-2 vaccine; Vaccine effectiveness.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- WHO. WHO Coronavirus (COVID-19) Dashboard; 2022. <https://covid19.who.int/>.
-
- McGill, COVID19 Vaccine Tracker Team. Egypt-COVID19 Vaccine Tracker; 2022. <https://covid19.trackvaccines.org/country/egypt/>.
-
- Gomaa M.R., El Rifay A.S., Shehata M., Kandeil A., Nabil Kamel M., Marouf M.A., et al. Incidence, household transmission, and neutralizing antibody seroprevalence of Coronavirus Disease 2019 in Egypt: results of a community-based cohort. PLoS Pathog. 2021;17(3):e1009413. doi: 10.1371/journal.ppat.1009413. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous