Association of FXI activity with thrombo-inflammation, extracellular matrix, lipid metabolism and apoptosis in venous thrombosis
- PMID: 35697739
- PMCID: PMC9192691
- DOI: 10.1038/s41598-022-13174-5
Association of FXI activity with thrombo-inflammation, extracellular matrix, lipid metabolism and apoptosis in venous thrombosis
Abstract
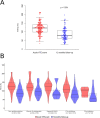
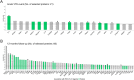
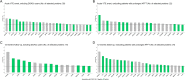
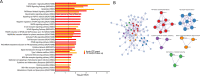
Animal experiments and early phase human trials suggest that inhibition of factor XIa (FXIa) safely prevents venous thromboembolism (VTE), and specific murine models of sepsis have shown potential efficacy in alleviating cytokine storm. These latter findings support the role of FXI beyond coagulation. Here, we combine targeted proteomics, machine learning and bioinformatics, to discover associations between FXI activity (FXI:C) and the plasma protein profile of patients with VTE. FXI:C was measured with a modified activated partial prothrombin time (APTT) clotting time assay. Proximity extension assay-based protein profiling was performed on plasma collected from subjects from the Genotyping and Molecular Phenotyping of Venous Thromboembolism (GMP-VTE) Project, collected during an acute VTE event (n = 549) and 12-months after (n = 187). Among 444 proteins investigated, N = 21 and N = 66 were associated with FXI:C during the acute VTE event and at 12 months follow-up, respectively. Seven proteins were identified as FXI:C-associated at both time points. These FXI-related proteins were enriched in immune pathways related to causes of thrombo-inflammation, extracellular matrix interaction, lipid metabolism, and apoptosis. The results of this study offer important new avenues for future research into the multiple properties of FXI, which are of high clinical interest given the current development of FXI inhibitors.
© 2022. The Author(s).
Conflict of interest statement
The GMP-VTE project was funded by the German Federal Ministry of Education and Research (BMBF 01EO1003), internal funds of the Clinical Epidemiology and Systems Medicine (Center for Thrombosis and Hemostasis), and a grant from Bayer AG. APR received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant agreement No. 813409. VTC, AS, JHP, SR, TK, MPN, HJS, CFO, HS, CEK, KJL, TM and MAN declare no conflicts of interest. SH, SS and KL are employees of Bayer AG. SVK reports grants and personal fees from Bayer AG, during the conduct of the study; grants from Boehringer Ingelheim, grants and personal fees from Actelion, grants and personal fees from Daiichi-Sankyo, grants and personal fees from BTG, personal fees from MSD and personal fees from Servier, outside the submitted work. HtC reports grants from Bayer and Pfizer. HTC is a stakeholder in Coagulation Profile and consultant for Alveron and has served on advisory boards for Bayer, Pfizer. PSW reports grants from Bayer AG and from the German Federal Ministry of Education and Research, during the conduct of the study; grants and personal fees from Boehringer Ingelheim, grants from Philips Medical Systems, grants and personal fees from Sanofi-Aventis, grants and personal fees from Bayer Vital, grants from Daiichi Sankyo Europe, personal fees from Bayer Health Care, personal fees from Astra Zeneca, personal fees and non-financial support from Diasorin and non-financial support from I.E.M., outside the submitted work.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

