Regional infusion of a class C TLR9 agonist enhances liver tumor microenvironment reprogramming and MDSC reduction to improve responsiveness to systemic checkpoint inhibition
- PMID: 35697801
- PMCID: PMC9750861
- DOI: 10.1038/s41417-022-00484-z
Regional infusion of a class C TLR9 agonist enhances liver tumor microenvironment reprogramming and MDSC reduction to improve responsiveness to systemic checkpoint inhibition
Abstract
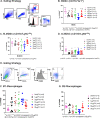
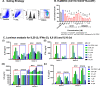
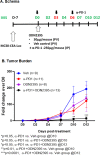
Myeloid-derived suppressor cells (MDSCs) expand in response to malignancy and suppress responsiveness to immunotherapy, including checkpoint inhibitors (CPIs). Within the liver, MDSCs have unique immunosuppressive features. While TLR9 agonists have shown promising activities in enhancing CPI responsiveness in superficial tumors amenable to direct needle injection, clinical success for liver tumors with TLR9 agonists has been limited by delivery challenges. Here, we report that regional intravascular infusion of ODN2395 into mice with liver metastasis (LM) partially eliminated liver MDSCs and reprogrammed residual MDSC. TLR9 agonist regional infusion also induced an increase in the M1/M2 macrophage ratio. Enhanced TLR9 signaling was demonstrated by an increased activation of in NFκB (pP65) and production of IL6 compared with systemic infusion. Further, PBMC-derived human MDSCs express TLR9, and treatment with class C TLR9 agonists (ODN2395 and SD101) reduced the expansion of MDSC population. TLR9 stimulation induced MDSC apoptosis and increased the M1/M2 macrophage ratio. Regional TLR9 agonist infusion along with systemic anti-PD-1 therapy improved control of LM. With effective delivery, TLR9 agonists have the potential to favorably reprogram the liver TME through reduction of MDSCs and favorable macrophage polarization, which may improve responsiveness to systemic CPI therapy.
© 2022. The Author(s).
Conflict of interest statement
SCK was a paid member of the scientific advisory board and received grant funding from TriSalus™ Life Sciences, Inc during the conduct of the research and initial drafting, and currently is employed by the company. BFC, CCG, JL, and PG are currently employed by TriSalus™ Life Sciences, Inc. The other authors have declared that no conflict of interest exists.
Figures




References
-
- Blum ES, Yang J, Komatsubara KM, Carvajal RD. Clinical management of uveal and conjunctival melanoma. Oncol (Williston Park) 2016;30:29–32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

