Genetic analysis of right heart structure and function in 40,000 people
- PMID: 35697867
- PMCID: PMC10313645
- DOI: 10.1038/s41588-022-01090-3
Genetic analysis of right heart structure and function in 40,000 people
Abstract
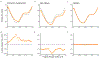
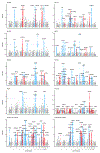
Congenital heart diseases often involve maldevelopment of the evolutionarily recent right heart chamber. To gain insight into right heart structure and function, we fine-tuned deep learning models to recognize the right atrium, right ventricle and pulmonary artery, measuring right heart structures in 40,000 individuals from the UK Biobank with magnetic resonance imaging. Genome-wide association studies identified 130 distinct loci associated with at least one right heart measurement, of which 72 were not associated with left heart structures. Loci were found near genes previously linked with congenital heart disease, including NKX2-5, TBX5/TBX3, WNT9B and GATA4. A genome-wide polygenic predictor of right ventricular ejection fraction was associated with incident dilated cardiomyopathy (hazard ratio, 1.33 per standard deviation; P = 7.1 × 10-13) and remained significant after accounting for a left ventricular polygenic score. Harnessing deep learning to perform large-scale cardiac phenotyping, our results yield insights into the genetic determinants of right heart structure and function.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
J.P.P. has served as a consultant for Maze Therapeutics. P.B. is supported by grants from Bayer AG and IBM applying machine learning in cardiovascular disease. S.A.L. receives sponsored research support from Bristol Myers Squibb / Pfizer, Bayer AG, Boehringer Ingelheim, and Fitbit, and has consulted for Bristol Myers Squibb / Pfizer and Bayer AG, and participates in a research collaboration with IBM. K.N. is employed by IBM Research. J.E.H. is supported by a grant from Bayer AG focused on machine-learning and cardiovascular disease and a research grant from Gilead Sciences. J.E.H. has received research supplies from EcoNugenics. A.A.P. is employed as a Venture Partner at GV; he is also supported by a grant from Bayer AG to the Broad Institute focused on machine learning for clinical trial design. P.T.E. received sponsored research support from Bayer AG and IBM Research. P.T.E. has also served on advisory boards or consulted for Bayer AG, Quest Diagnostics, MyoKardia and Novartis. The Broad Institute has filed for a patent on an invention from P.T.E., M.E.L., and J.P.P. related to a genetic risk predictor for aortic disease. All remaining authors report no competing interests.
Figures
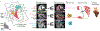



References
-
- Farmer CG Evolution of the vertebrate cardio-pulmonary system. Annual Review of Physiology 61, 573–592 (1999). - PubMed
-
- Galli D et al. Atrial myocardium derives from the posterior region of the second heart field, which acquires left-right identity as Pitx2c is expressed. Development 135, 1157–1167 (2008). - PubMed
-
- Meilhac SM & Buckingham ME The deployment of cell lineages that form the mammalian heart. Nature Reviews Cardiology 15, 705–724 (2018). - PubMed
Methods-only References
-
- He K, Zhang X, Ren S & Sun J Deep residual learning for image recognition. arXiv:1512.03385 [cs] (2015).
-
- Krizhevsky A, Sutskever I & Hinton GE ImageNet classification with deep convolutional neural networks. Commun. ACM 60, 84–90 (2017).
-
- Kingma DP & Ba J Adam: a method for stochastic optimization. arXiv:1412.6980 [cs] (2017).
-
- Smith LN Cyclical learning rates for training neural networks. arXiv:1506.01186 [cs] (2015).
Publication types
MeSH terms
Grants and funding
- R01 HL139731/HL/NHLBI NIH HHS/United States
- R01 HL141434/HL/NHLBI NIH HHS/United States
- R01 HL148050/HL/NHLBI NIH HHS/United States
- T32 HL007208/HL/NHLBI NIH HHS/United States
- MC_PC_17228/MRC_/Medical Research Council/United Kingdom
- T32 HL007604/HL/NHLBI NIH HHS/United States
- T32 HL094301/HL/NHLBI NIH HHS/United States
- K08 HL159346/HL/NHLBI NIH HHS/United States
- K24 HL105780/HL/NHLBI NIH HHS/United States
- R01 HL140224/HL/NHLBI NIH HHS/United States
- R01 HL092577/HL/NHLBI NIH HHS/United States
- MC_QA137853/MRC_/Medical Research Council/United Kingdom
- U54 HL120163/HL/NHLBI NIH HHS/United States
- R01 HL128914/HL/NHLBI NIH HHS/United States
- U01 HG011719/HG/NHGRI NIH HHS/United States
- R01 HL127564/HL/NHLBI NIH HHS/United States
- K24 HL153669/HL/NHLBI NIH HHS/United States
- R01 HL134893/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

