Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts
- PMID: 35697880
- PMCID: PMC9552938
- DOI: 10.1007/s00401-022-02444-1
Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts
Abstract
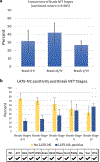
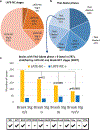
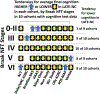
Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) and Alzheimer's disease neuropathologic change (ADNC) are each associated with substantial cognitive impairment in aging populations. However, the prevalence of LATE-NC across the full range of ADNC remains uncertain. To address this knowledge gap, neuropathologic, genetic, and clinical data were compiled from 13 high-quality community- and population-based longitudinal studies. Participants were recruited from United States (8 cohorts, including one focusing on Japanese-American men), United Kingdom (2 cohorts), Brazil, Austria, and Finland. The total number of participants included was 6196, and the average age of death was 88.1 years. Not all data were available on each individual and there were differences between the cohorts in study designs and the amount of missing data. Among those with known cognitive status before death (n = 5665), 43.0% were cognitively normal, 14.9% had MCI, and 42.4% had dementia-broadly consistent with epidemiologic data in this age group. Approximately 99% of participants (n = 6125) had available CERAD neuritic amyloid plaque score data. In this subsample, 39.4% had autopsy-confirmed LATE-NC of any stage. Among brains with "frequent" neuritic amyloid plaques, 54.9% had comorbid LATE-NC, whereas in brains with no detected neuritic amyloid plaques, 27.0% had LATE-NC. Data on LATE-NC stages were available for 3803 participants, of which 25% had LATE-NC stage > 1 (associated with cognitive impairment). In the subset of individuals with Thal Aβ phase = 0 (lacking detectable Aβ plaques), the brains with LATE-NC had relatively more severe primary age-related tauopathy (PART). A total of 3267 participants had available clinical data relevant to frontotemporal dementia (FTD), and none were given the clinical diagnosis of definite FTD nor the pathological diagnosis of frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP). In the 10 cohorts with detailed neurocognitive assessments proximal to death, cognition tended to be worse with LATE-NC across the full spectrum of ADNC severity. This study provided a credible estimate of the current prevalence of LATE-NC in advanced age. LATE-NC was seen in almost 40% of participants and often, but not always, coexisted with Alzheimer's disease neuropathology.
Keywords: ACT; ADRD; APOE; Biobank for aging studies; CC75C; CFAS; Epidemiology; HAAS; Mayo clinic study of aging; NFT; Nondemented; Nun study; Oldest-old; ROS-MAP; Tau; The 90 + study; VITA; Vantaa 85 +.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Figures




References
-
- (2017) https://www.ssa.gov/oact/STATS/table4c6.html. Accessed 14 Dec 2021
-
- (2021) Provisional life expectancy estimates for January through June, 2020. https://www.cdc.gov/nchs/data/vsrr/VSRR10-508.pdf. Accessed 14 Dec 2021
Publication types
MeSH terms
Substances
Grants and funding
- K08 AG065463/AG/NIA NIH HHS/United States
- R01 AG061111/AG/NIA NIH HHS/United States
- R01 AG054449/AG/NIA NIH HHS/United States
- RF1 NS118584/NS/NINDS NIH HHS/United States
- U.1052.00.0013/MRC_/Medical Research Council/United Kingdom
- R01 AG062517/AG/NIA NIH HHS/United States
- P30 AG066519/AG/NIA NIH HHS/United States
- UF1 NS125417/NS/NINDS NIH HHS/United States
- G0601022/MRC_/Medical Research Council/United Kingdom
- P30 AG010161/AG/NIA NIH HHS/United States
- R01 AG038651/AG/NIA NIH HHS/United States
- R01 AG064233/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- MRC/G0900582/MRC_/Medical Research Council/United Kingdom
- R01 AG022018/AG/NIA NIH HHS/United States
- U01 AG006786/AG/NIA NIH HHS/United States
- R01 AG057187/AG/NIA NIH HHS/United States
- P30 AG072975/AG/NIA NIH HHS/United States
- G0900582/MRC_/Medical Research Council/United Kingdom
- RF1 AG069052/AG/NIA NIH HHS/United States
- P30 AG072946/AG/NIA NIH HHS/United States
- R01 AG021055/AG/NIA NIH HHS/United States
- R01 AG034676/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- R01 AG067482/AG/NIA NIH HHS/United States
- RF1 AG072080/AG/NIA NIH HHS/United States
- K24 AG053435/AG/NIA NIH HHS/United States
- R33 AG058738/AG/NIA NIH HHS/United States
- UF1 AG057707/AG/NIA NIH HHS/United States
- P30 AG072977/AG/NIA NIH HHS/United States
- K08 AG065426/AG/NIA NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- U19 AG069701/AG/NIA NIH HHS/United States
- P30 AG072958/AG/NIA NIH HHS/United States
- MRC/G9901400/MRC_/Medical Research Council/United Kingdom
- P30 AG072972/AG/NIA NIH HHS/United States
- G9901400/MRC_/Medical Research Council/United Kingdom
- U19 AG066567/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

