Deletion of both Dectin-1 and Dectin-2 affects the bacterial but not fungal gut microbiota and susceptibility to colitis in mice
- PMID: 35698210
- PMCID: PMC9195441
- DOI: 10.1186/s40168-022-01273-4
Deletion of both Dectin-1 and Dectin-2 affects the bacterial but not fungal gut microbiota and susceptibility to colitis in mice
Abstract
Background: Innate immunity genes have been reported to affect susceptibility to inflammatory bowel diseases (IBDs) and colitis in mice. Dectin-1, a receptor for fungal cell wall β-glucans, has been clearly implicated in gut microbiota modulation and modification of the susceptibility to gut inflammation. Here, we explored the role of Dectin-1 and Dectin-2 (another receptor for fungal cell wall molecules) deficiency in intestinal inflammation.
Design: Susceptibility to dextran sodium sulfate (DSS)-induced colitis was assessed in wild-type, Dectin-1 knockout (KO), Dectin-2KO, and double Dectin-1KO and Dectin-2KO (D-1/2KO) mice. Inflammation severity, as well as bacterial and fungal microbiota compositions, was monitored.
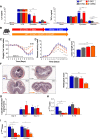
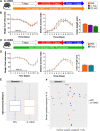
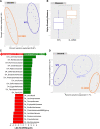
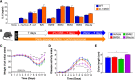
Results: While deletion of Dectin-1 or Dectin-2 did not have a strong effect on DSS-induced colitis, double deletion of Dectin-1 and Dectin-2 significantly protected the mice from colitis. The protection was largely mediated by the gut microbiota, as demonstrated by fecal transfer experiments. Treatment of D-1/2KO mice with opportunistic fungal pathogens or antifungal agents did not affect the protection against gut inflammation, suggesting that the fungal microbiota had no role in the protective phenotype. Amplicon-based microbiota analysis of the fecal bacterial and fungal microbiota of D-1/2KO mice confirmed the absence of changes in the mycobiota but strong modification of the bacterial microbiota. We showed that bacteria from the Lachnospiraceae family were at least partly involved in this protection and that treatment with Blautia hansenii was enough to recapitulate the protection.
Conclusions: Deletion of both the Dectin-1 and Dectin-2 receptors triggered a global shift in the microbial gut environment, affecting, surprisingly, mainly the bacterial population and driving protective effects in colitis. Members of the Lachnospiraceae family seem to play a central role in this protection. These findings provide new insights into the role of the Dectin receptors, which have been described to date as affecting only the fungal population, in intestinal physiopathology and in IBD. Video Abstract.
Keywords: Dectin-1; Dectin-2; Gut inflammation; Immune response; Microbiota.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

