Protected Poly(3-sulfopropyl methacrylate) Copolymers: Synthesis, Stability, and Orthogonal Deprotection
- PMID: 35698473
- PMCID: PMC9185742
- DOI: 10.1021/acspolymersau.1c00044
Protected Poly(3-sulfopropyl methacrylate) Copolymers: Synthesis, Stability, and Orthogonal Deprotection
Abstract
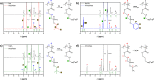
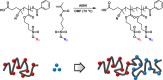
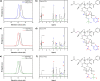
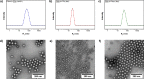
Because of their permanent charge, strong polyelectrolytes remain challenging to characterize, in particular, when they are combined with hydrophobic features. For this reason, they are typically prepared through a postmodification of a fully hydrophobic precursor. Unfortunately, these routes often result in an incomplete functionalization or otherwise require harsh reaction conditions, thus limiting their applicability. To overcome these problems, in this work a strategy is presented that facilitates the preparation of well-defined strong polyanions by starting from protected 3-sulfopropyl methacrylate monomers. Depending on the chemistry of the protecting group, the hydrophobic precursor could be quantitatively converted into a strong polyanion under nucleophilic, acidic, or basic conditions. As a proof of concept, orthogonally protected diblock copolymers were synthesized, selectively deprotected, and allowed to self-assemble in aqueous solution. Further conversion into a fully water-soluble polyanion was achieved by deprotecting the second block as well.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Förster S.; Schmidt M. Polyelectrolytes in Solution. Adv. Polym. Sci. 1995, 120, 51–133. 10.1007/3-540-58704-7_2. - DOI
-
- Li F.; Schellekens M.; de Bont J.; Peters R.; Overbeek A.; Leermakers F. A. M.; Tuinier R. Self-Assembled Structures of PMAA-PMMA Block Copolymers: Synthesis, Characterization, and Self-Consistent Field Computations. Macromolecules 2015, 48, 1194–1203. 10.1021/ma501878n. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
