Metal complexes of thiosemicarbazones derived by 2-quinolones with Cu(I), Cu(II) and Ni(II); Identification by NMR, IR, ESI mass spectra and in silico approach as potential tools against SARS-CoV-2
- PMID: 35698532
- PMCID: PMC9179108
- DOI: 10.1016/j.molstruc.2022.133480
Metal complexes of thiosemicarbazones derived by 2-quinolones with Cu(I), Cu(II) and Ni(II); Identification by NMR, IR, ESI mass spectra and in silico approach as potential tools against SARS-CoV-2
Abstract
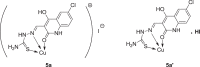
Substituted thiosemicarbazones derived by 2-quinolone were synthesized to investigate their complexation capability towards Cu(I), Cu(II) and Ni(II) salts. The structure of the complexes was established by ESI, IR and NMR spectra in addition to elemental analyses. Monodetate Cu(I) quinoloyl-substituted ligands were observed, whereas Ni(II) and Cu(II) formed bidentate-thiosemicarbazone derived by 2-quinolones. Subsequently, molecular docking was used to evaluate each analog's binding affinity as well as the inhibition constant (ki) to RdRp complex of SARS-CoV-2. Docking results supported the ability of the tested complexes that potentially inhibit the RdRp of SARSCov-2 show binding energy higher than their corresponding ligands. Additionally, ADMET prediction revealed that some compounds stratify to Lipinski's rule, indicating a good oral absorption, high bioavailability good permeability, and transport via biological membranes. Therefore, these metals-based complexes are suggested to be potentially good candidates as anti-covid agents.
Keywords: 2-Quinolones; Anti-Covid agents; Bidentate; Cu(I); ESI; Molecular docking; Monodentate; NMR; Ni (II) and Cu(II) salts; Thiosemicarbazones.
© 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
I would like to confirm that the my MS entitled Metal complexes of thiosemicarbazones derived by 2-quinolones with Cu(I), Cu(II) and Ni(II); identification by NMR, IR, ESI mass spectra and in silico approach as potential tools against SARS-CoV-2 has no declaration of interest.
Figures










Similar articles
-
Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)-A Review.Molecules. 2023 Feb 14;28(4):1808. doi: 10.3390/molecules28041808. Molecules. 2023. PMID: 36838796 Free PMC article. Review.
-
Spectroscopic and biological approach of Ni(II), Cu(II) and Co(II) complexes of 4-methoxy/ethoxybenzaldehyde thiosemicarbazone glyoxime.Spectrochim Acta A Mol Biomol Spectrosc. 2014;121:205-15. doi: 10.1016/j.saa.2013.10.040. Epub 2013 Oct 25. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24239764
-
New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study.Molecules. 2021 Apr 15;26(8):2288. doi: 10.3390/molecules26082288. Molecules. 2021. PMID: 33920893 Free PMC article.
-
Copper(II) complexes based on thiosemicarbazone ligand: Preparation, crystal structure, Hirshfeld surface, energy framework, antiMycobacterium activity, in silico and molecular docking studies.J Inorg Biochem. 2021 Oct;223:111543. doi: 10.1016/j.jinorgbio.2021.111543. Epub 2021 Jul 15. J Inorg Biochem. 2021. PMID: 34298306
-
Antifungal and antitumor activity of heterocyclic thiosemicarbazones and their metal complexes: current status.Biometals. 1992 Summer;5(2):121-6. doi: 10.1007/BF01062223. Biometals. 1992. PMID: 1525478 Review.
Cited by
-
Copper Complexes of 1,4-Naphthoquinone Containing Thiosemicarbazide and Triphenylphosphine Oxide Moieties; Synthesis and Identification by NMR, IR, Mass, UV Spectra, and DFT Calculations.ACS Omega. 2022 Sep 16;7(38):34463-34475. doi: 10.1021/acsomega.2c04113. eCollection 2022 Sep 27. ACS Omega. 2022. PMID: 36188271 Free PMC article.
-
Exploring the antimalarial and antioxidant efficacy of transition metal(II) chelates of thiosemicarbazone ligands: spectral investigations, molecular docking, DFT, MESP and ADMET.Biometals. 2024 Feb;37(1):247-265. doi: 10.1007/s10534-023-00546-1. Epub 2023 Nov 8. Biometals. 2024. PMID: 37938497
-
Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)-A Review.Molecules. 2023 Feb 14;28(4):1808. doi: 10.3390/molecules28041808. Molecules. 2023. PMID: 36838796 Free PMC article. Review.
-
Synthesis, characterization, molecular docking studies, and theoretical calculations of novel Ni (II), Cu (II), and Zn (II) complexes based on benzothiazole derivative.BMC Chem. 2025 Jul 4;19(1):202. doi: 10.1186/s13065-025-01576-1. BMC Chem. 2025. PMID: 40616156 Free PMC article.
References
-
- Beraldo H., Gambino D. Mini-Rev. The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Med. Chem. 2004;4:31–39. - PubMed
-
- Quenelle D.C., Keith K.A., Kern E.R. In vitro and in vivo evaluation of isatin-beta-thiosemicarbazone and marboran against vaccinia and cowpox virus infections. Antivir. Res. 2006;71:24–30. - PubMed
-
- Molter A., Rust J., Lehmann C.W., Deepa G., Chiba P., Mohr F. Synthesis, structures and anti-malaria activity of some gold(i) phosphine complexes containing seleno- and thiosemicarbazonato ligands. Dalton Trans. 2011;40:9810–9820. - PubMed
-
- Arora S., Agarwal S., Singhal S. Anticancer activities of thiosemi-carbazides/thiosemicarbazones: a review. Int. J. Pharm. Pharm. Sci. 2014;6(9):34–41.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

