Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform
- PMID: 35698725
- PMCID: PMC9179144
- DOI: 10.1016/S2665-9913(22)00098-4
Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform
Abstract
Background: The risk of severe COVID-19 outcomes in people with immune-mediated inflammatory diseases and on immune-modifying drugs might not be fully mediated by comorbidities and might vary by factors such as ethnicity. We aimed to assess the risk of severe COVID-19 in adults with immune-mediated inflammatory diseases and in those on immune-modifying therapies.
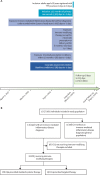
Methods: We did a cohort study, using OpenSAFELY (an analytics platform for electronic health records) and TPP (a software provider for general practitioners), analysing routinely collected primary care data linked to hospital admission, death, and previously unavailable hospital prescription data. We included people aged 18 years or older on March 1, 2020, who were registered with TPP practices with at least 12 months of primary care records before March, 2020. We used Cox regression (adjusting for confounders and mediators) to estimate hazard ratios (HRs) comparing the risk of COVID-19-related death, critical care admission or death, and hospital admission (from March 1 to Sept 30, 2020) in people with immune-mediated inflammatory diseases compared with the general population, and in people with immune-mediated inflammatory diseases on targeted immune-modifying drugs (eg, biologics) compared with those on standard systemic treatment (eg, methotrexate).
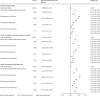
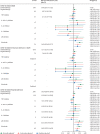
Findings: We identified 17 672 065 adults; 1 163 438 adults (640 164 [55·0%] women and 523 274 [45·0%] men, and 827 457 [71·1%] of White ethnicity) had immune-mediated inflammatory diseases, and 16 508 627 people (8 215 020 [49·8%] women and 8 293 607 [50·2%] men, and 10 614 096 [64·3%] of White ethnicity) were included as the general population. Of 1 163 438 adults with immune-mediated inflammatory diseases, 19 119 (1·6%) received targeted immune-modifying therapy and 181 694 (15·6%) received standard systemic therapy. Compared with the general population, adults with immune-mediated inflammatory diseases had an increased risk of COVID-19-related death after adjusting for confounders (age, sex, deprivation, and smoking status; HR 1·23, 95% CI 1·20-1·27) and further adjusting for mediators (body-mass index [BMI], cardiovascular disease, diabetes, and current glucocorticoid use; 1·15, 1·11-1·18). Adults with immune-mediated inflammatory diseases also had an increased risk of COVID-19-related critical care admission or death (confounder-adjusted HR 1·24, 95% CI 1·21-1·28; mediator-adjusted 1·16, 1·12-1·19) and hospital admission (confounder-adjusted 1·32, 1·29-1·35; mediator-adjusted 1·20, 1·17-1·23). In post-hoc analyses, the risk of severe COVID-19 outcomes in people with immune-mediated inflammatory diseases was higher in non-White ethnic groups than in White ethnic groups (as it was in the general population). We saw no evidence of increased COVID-19-related death in adults on targeted, compared with those on standard systemic, therapy after adjusting for confounders (age, sex, deprivation, BMI, immune-mediated inflammatory diseases [bowel, joint, and skin], cardiovascular disease, cancer [excluding non-melanoma skin cancer], stroke, and diabetes (HR 1·03, 95% CI 0·80-1·33), and after additionally adjusting for current glucocorticoid use (1·01, 0·78-1·30). There was no evidence of increased COVID-19-related death in adults prescribed tumour necrosis factor inhibitors, interleukin (IL)-12/IL‑23 inhibitors, IL-17 inhibitors, IL-6 inhibitors, or Janus kinase inhibitors compared with those on standard systemic therapy. Rituximab was associated with increased COVID-19-related death (HR 1·68, 95% CI 1·11-2·56), with some attenuation after excluding people with haematological malignancies or organ transplants (1·54, 0·95-2·49).
Interpretation: COVID-19 deaths and hospital admissions were higher in people with immune-mediated inflammatory diseases. We saw no increased risk of adverse COVID-19 outcomes in those on most targeted immune-modifying drugs for immune-mediated inflammatory diseases compared with those on standard systemic therapy.
Funding: UK Medical Research Council, NIHR Biomedical Research Centre at King's College London and Guy's and St Thomas' NHS Foundation Trust, and Wellcome Trust.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
BG has received research funding from the Laura and John Arnold Foundation, the UK National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK (HDRUK), the Health Foundation, WHO, UK Research and Innovation (UKRI), Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he also receives personal income from speaking and writing for lay audiences on the misuse of science and is a non-executive director of NHS Digital. CHS received departmental research funding from AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Leo, Pfizer, Novartis, Regeneron, SwedishOrphan Biovitrum, and Roche, and is an investigator within consortia that have industry partners. JG has received honoraria from AbbVie, Amgen, Celgene, Chugai, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sobi, and UCB, and has research funding from Amgen, AstraZeneca, Gilead, Janssen, Medicago, Novovax, and Pfizer. MY has received honoraria from AbbVie and UCB. CWL has received honoraria from AbbVie, Bristol Myers Squibb, Celltrion, Ferring, Galapagos, Gilead, GlaxoSmithKline, Iterative Scopes, Janssen, Fresnius Kabi, Dr Falk, Vifor Pharma, Pfizer, Takeda, and Trellus Health. CHS and SML have received grants from the Horizon 2020 European Commission-funded consortium, which has industry partners involved in manufacture of treatments for immune-mediated inflammatory diseases (see the Biomap website for complete listing). EW has received payment from AstraZeneca for providing a training session, unrelated to the current manuscript. KEM has received consulting fees from Amgen. RM has received consulting fees from Amgen. LAT has received consulting fees from Bayer (payed to the institution), support for attending Medicines and Healthcare products Regulatory Agency meetings and is a member of two non-industry-funded trial advisory committees (unpaid). SN has received grants from Pfizer and honoraria for delivering educational presentations from Pfizer and Janssen. JB is funded by a studentship from GlaxoSmithKline. HIM was an occasional invited expert to the COVID-19 Vaccines Safety Surveillance Methodologies Expert Working Group, which has now come to a close. NAK has received departmental research funding from AbbVie, Biogen, Celgene, Celtrion, Galapagos, Merck Sharp & Dohme, Napp, Pfizer, Pharmacosmos, Roche, and Takeda; consulting fees from Amgen, Bristol Myers Squibb, Dr Falk, Janssen, Mylan, Pharmacosmos, Galapagos, Takeda, and Tillotts; honoraria from Allergan, Celltrion, Dr Falk, Ferring, Janssen, Pharmacosmos, Takeda, Tilllotts, and Galapagos; and support for meetings or travel from AbbVie, Dr Falk, and Janssen. All other authors declare no competing interests.
Figures



References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

