Pulmonary and Systemic Pathology in COVID-19—Holistic Pathological Analyses
- PMID: 35698804
- PMCID: PMC9549895
- DOI: 10.3238/arztebl.m2022.0231
Pulmonary and Systemic Pathology in COVID-19—Holistic Pathological Analyses
Abstract
Background: The COVID-19 pandemic is the third worldwide coronavirus-associated disease outbreak in the past 20 years. Lung involvement, with acute respiratory distress syndrome (ARDS) in severe cases, is the main clinical feature of this disease; the cardiovascular system, the central nervous system, and the gastrointestinal tract can also be affected. The pathophysiology of both pulmonary and extrapulmonary organ damage was almost completely unknown when the pandemic began.
Methods: This review is based on pertinent publications retrieved by a selective search concerning the structural changes and pathophysiology of COVID-19, with a focus on imaging techniques.
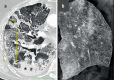
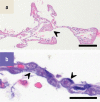
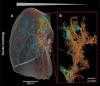
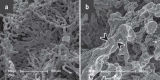
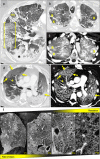
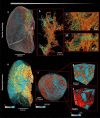
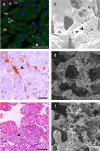
Results: Immunohistochemical, electron-microscopic and molecular pathological analyses of tissues obtained by autopsy have improved our understanding of COVID-19 pathophysiology, including molecular regulatory mechanisms. Intussusceptive angiogenesis (IA) has been found to be a prominent pattern of damage in the affected organs of COVID-19 patients. In IA, an existing vessel changes by invagination of the endothelium and formation of an intraluminal septum, ultimately giving rise to two new lumina. This alters hemodynamics within the vessel, leading to a loss of laminar flow and its replacement by turbulent, inhomogeneous flow. IA, which arises because of ischemia due to thrombosis, is itself a risk factor for the generation of further microthrombi; these have been detected in the lungs, heart, liver, kidneys, brain, and placenta of COVID-19 patients.
Conclusion: Studies of autopsy material from various tissues of COVID-19 patients have revealed ultrastructural evidence of altered microvascularity, IA, and multifocal thrombi. These changes may contribute to the pathophysiology of post-acute interstitial fibrotic organ changes as well as to the clinical picture of long COVID.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

