Patient characteristics associated with conversion from negative to positive severe acute respiratory syndrome coronavirus-2 polymerase chain reaction test results: Implications for clinical false-negativity from a single-center: A case-control study
- PMID: 35698816
- PMCID: PMC9350093
- DOI: 10.1002/jmv.27932
Patient characteristics associated with conversion from negative to positive severe acute respiratory syndrome coronavirus-2 polymerase chain reaction test results: Implications for clinical false-negativity from a single-center: A case-control study
Abstract
Background: Accurate diagnosis of coronavirus disease 2019 is essential to limiting transmission within healthcare settings. The aim of this study was to identify patient demographic and clinical characteristics that could impact the clinical sensitivity of the nasopharyngeal severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) reverse transcription polymerase chain reaction (RT-PCR) test.
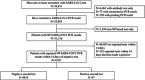
Methods: We conducted a retrospective, matched case-control study of patients who underwent repeated nasopharyngeal SARS-CoV2 RT-PCR testing at a tertiary care academic medical center between March 1 and July 23, 2020. The primary endpoint was conversion from negative to positive PCR status within 14 days. We conducted conditional logistic regression modeling to assess the associations between demographic and clinical features and conversion to test positivity.
Results: Of 51,116 patients with conclusive SARS-CoV2 nasopharyngeal RT-PCR results, 97 patients converted from negative to positive within 14 days. We matched those patients 1:2 to 194 controls by initial test date. In multivariate analysis, clinical suspicion for a respiratory infection (adjusted odds ratio [aOR] 20.9, 95% confidence interval [CI]: 3.1-141.2) and lack of pulmonary imaging (aOR 4.7, 95% CI: 1.03-21.8) were associated with conversion, while a lower burden of comorbidities trended toward an increased odds of conversion (aOR 2.2, 95% CI: 0.9-5.3).
Conclusions: Symptoms consistent with a respiratory infection, especially in relatively healthy individuals, should raise concerns about a clinical false-negative result. We have identified several characteristics that should be considered when creating institutional infection prevention guidelines in the absence of more definitive data and should be included in future studies.
Keywords: COVID-19; SARS-CoV2 RT-PCR; clinical sensitivity.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Similar articles
-
Association of Nirmatrelvir/Ritonavir Treatment on Upper Respiratory Severe Acute Respiratory Syndrome Coronavirus 2 Reverse Transcription-Polymerase Chain Reaction (SARS-Cov-2 RT-PCR) Negative Conversion Rates Among High-Risk Patients With Coronavirus Disease 2019 (COVID-19).Clin Infect Dis. 2023 Feb 8;76(3):e148-e154. doi: 10.1093/cid/ciac600. Clin Infect Dis. 2023. PMID: 35870128 Free PMC article.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
-
Clinical characteristics of hospitalized patients with false-negative severe acute respiratory coronavirus virus 2 (SARS-CoV-2) test results.Infect Control Hosp Epidemiol. 2022 Apr;43(4):467-473. doi: 10.1017/ice.2021.146. Epub 2021 Apr 19. Infect Control Hosp Epidemiol. 2022. PMID: 33867000 Free PMC article.
-
Predictors of negative first SARS-CoV-2 RT-PCR despite final diagnosis of COVID-19 and association with outcome.Sci Rep. 2021 Jan 27;11(1):2388. doi: 10.1038/s41598-021-82192-6. Sci Rep. 2021. PMID: 33504923 Free PMC article.
-
False-negative results of initial RT-PCR assays for COVID-19: A systematic review.PLoS One. 2020 Dec 10;15(12):e0242958. doi: 10.1371/journal.pone.0242958. eCollection 2020. PLoS One. 2020. PMID: 33301459 Free PMC article.
Cited by
-
Associations between genetic mutations in different SARS-CoV-2 strains and negative conversion time of viral RNA among imported cases in Hangzhou: A cross-sectional study.Virus Res. 2024 Jul;345:199400. doi: 10.1016/j.virusres.2024.199400. Epub 2024 May 20. Virus Res. 2024. PMID: 38763300 Free PMC article.
References
-
- False‐Negative Results of Initial RT‐PCR Assays for COVID‐19: A Systematic Review; 2021. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0242958 - PMC - PubMed
-
- Lippi G, Simundic A‐M, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID‐19). Clin Chem Lab Med. 2020;58(7):1070‐1076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous