Synthesis and biological evaluation of thieno[3,2- c]pyrazol-3-amine derivatives as potent glycogen synthase kinase 3β inhibitors for Alzheimer's disease
- PMID: 35698879
- PMCID: PMC9225722
- DOI: 10.1080/14756366.2022.2086867
Synthesis and biological evaluation of thieno[3,2- c]pyrazol-3-amine derivatives as potent glycogen synthase kinase 3β inhibitors for Alzheimer's disease
Abstract
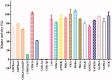
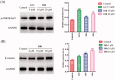
Glycogen synthase kinase 3β (GSK-3β) catalyses the hyperphosphorylation of tau protein in the Alzheimer's disease (AD) pathology. A series of novel thieno[3,2-c]pyrazol-3-amine derivatives were designed and synthesised and evaluated as potential GSK-3β inhibitors by structure-guided drug rational design approach. The thieno[3,2-c]pyrazol-3-amine derivative 16b was identified as a potent GSK-3β inhibitor with an IC50 of 3.1 nM in vitro and showed accepted kinase selectivity. In cell levels, 16b showed no toxicity on the viability of SH-SY5Y cells at the concentration up to 50 μM and targeted GSK-3β with the increased phosphorylated GSK-3β at Ser9. Western blot analysis indicated that 16b decreased the phosphorylated tau at Ser396 in a dose-dependent way. Moreover, 16b effectively increased expressions of β-catenin as well as the GAP43, N-myc, and MAP-2, and promoted the differentiated neuronal neurite outgrowth. Therefore, the thieno[3,2-c]pyrazol-3-amine derivative 16b could serve as a promising GSK-3β inhibitor for the treatment of AD.
Keywords: neurite outgrowth; Alzheimer’s disease; Aβ; GSK-3β inhibitors; tau hyperphosphorylation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







Similar articles
-
Synthesis and biological evaluation of thieno[3,2-c]pyrazol-3-amine derivatives as potent glycogen synthase kinase 3β inhibitors for Alzheimer's disease.Bioorg Chem. 2023 Sep;138:106663. doi: 10.1016/j.bioorg.2023.106663. Epub 2023 Jun 13. Bioorg Chem. 2023. PMID: 37329814
-
Synthesis and biological evaluation of novel pyrrolo[2,3-b]pyridine derivatives as potent GSK-3β inhibitors for treating Alzheimer's disease.Eur J Med Chem. 2025 Mar 5;285:117236. doi: 10.1016/j.ejmech.2025.117236. Epub 2025 Jan 2. Eur J Med Chem. 2025. PMID: 39798400
-
A Unique GSK-3β inhibitor B10 Has a Direct Effect on Aβ, Targets Tau and Metal Dyshomeostasis, and Promotes Neuronal Neurite Outgrowth.Cells. 2020 Mar 7;9(3):649. doi: 10.3390/cells9030649. Cells. 2020. PMID: 32155989 Free PMC article.
-
Diabetes mellitus and Alzheimer's disease: GSK-3β as a potential link.Behav Brain Res. 2018 Feb 26;339:57-65. doi: 10.1016/j.bbr.2017.11.015. Epub 2017 Nov 21. Behav Brain Res. 2018. PMID: 29158110 Review.
-
Therapeutic implications of glycogen synthase kinase-3β in Alzheimer's disease: a novel therapeutic target.Int J Neurosci. 2024 Jun;134(6):603-619. doi: 10.1080/00207454.2022.2130297. Epub 2022 Oct 10. Int J Neurosci. 2024. PMID: 36178363 Review.
Cited by
-
Identification of a novel pyrrolo[2,3-b]pyridine compound as a potent glycogen synthase kinase 3β inhibitor for treating Alzheimer's disease.J Enzyme Inhib Med Chem. 2025 Dec;40(1):2466846. doi: 10.1080/14756366.2025.2466846. Epub 2025 Feb 20. J Enzyme Inhib Med Chem. 2025. PMID: 39976249 Free PMC article.
References
-
- Patterson C, World Alzheimer report 2018. London: Alzheimer’s Disease International; 2018.
-
- Srivastava S, Ahmad R, Khare SK.. Alzheimer's disease and its treatment by different approaches: A review. Eur J Med Chem 2021;216:1724. - PubMed
-
- Liu W, Liu X, Liu W, et al. . Discovery of novel β-carboline derivatives as selective AChE inhibitors with GSK-3β inhibitory property for the treatment of Alzheimer's disease. Eur J Med Chem 2022; 229:114095. - PubMed
-
- Verma A, Kumar Waiker D, Bhardwaj B, et al. . The molecular mechanism, targets, and novel molecules in the treatment of Alzheimer's disease. Bioorg Chem 2022;119:105562. - PubMed
-
- Syed YY. Sodium Oligomannate: First approval. Drugs 2020;80:441–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
