Effects of intravenous administration of peripheral blood-derived mesenchymal stromal cells after infusion of lipopolysaccharide in horses
- PMID: 35698909
- PMCID: PMC9308407
- DOI: 10.1111/jvim.16447
Effects of intravenous administration of peripheral blood-derived mesenchymal stromal cells after infusion of lipopolysaccharide in horses
Abstract
Background: A systemic and dysregulated immune response to infection contributes to morbidity and mortality associated with sepsis. Peripheral blood-derived mesenchymal stromal cells (PB-MSC) mitigate inflammation in animal models of sepsis. Allogeneic PB-MSC administered IV to horses is well-tolerated but therapeutic benefits are unknown.
Hypothesis: After IV lipopolysaccharide (LPS) infusion, horses treated with PB-MSC would have less severe clinical signs, clinicopathological abnormalities, inflammatory cytokine gene expression, and oxidative stress compared to controls administered a placebo.
Animals: Sixteen horses were included in this study.
Methods: A randomized placebo-controlled experimental trial was performed. Sixteen healthy horses were assigned to 1 of 2 treatment groups (1 × 109 PB-MSC or saline placebo). Treatments were administered 30 minutes after completion of LPS infusion of approximately 30 ng/kg. Clinical signs, clinicopathological variables, inflammatory cytokine gene expression, and oxidative stress markers were assessed at various time points over a 24-hour period.
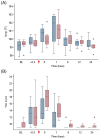
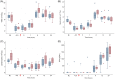
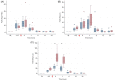
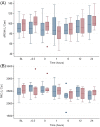
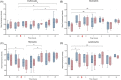
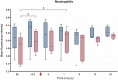
Results: A predictable response to IV LPS infusion was observed in all horses. At the dose administered, there was no significant effect of PB-MSC on clinical signs, clinicopathological variables, or inflammatory cytokine gene expression at any time point. Antioxidant potential was not different between treatment groups, but intracellular ROS increased over time in the placebo group. Other variables that changed over time were likely due to effects of IV LPS infusion.
Conclusions and clinical importance: Administration of allogeneic PB-MSC did not cause clinically detectable adverse effects in healthy horses. The dose of PB-MSC used here is unlikely to exert a beneficial effect in endotoxemic horses.
Keywords: allogeneic; endotoxemia; horses; oxidative stress.
© 2022 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
George E. Moore serves as Consulting Editor for Experimental Design and Statistics for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript. No other authors have a conflict of interest.
Figures
References
-
- Cohen ND. Causes of and farm management factors associated with disease and death in foals. J Am Vet Med Assoc. 1994;204:1644‐1651. - PubMed
-
- Peek SF, Semrad S, McGuirk SM, et al. Prognostic value of clinicopathologic variables obtained at admission and effect of antiendotoxin plasma on survival in septic and critically ill foals. J Vet Intern Med. 2006;20:569‐574. - PubMed
-
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644‐1655. - PubMed
-
- Werners AH, Bull S, Fink‐Gremmels J. Endotoxaemia: a review with implications for the horse. Equine Vet J. 2005;37:371‐383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

