Normobaric hyperoxia plays a neuroprotective role after cerebral ischemia by maintaining the redox homeostasis and the level of connexin43 in astrocytes
- PMID: 35698913
- PMCID: PMC9437237
- DOI: 10.1111/cns.13875
Normobaric hyperoxia plays a neuroprotective role after cerebral ischemia by maintaining the redox homeostasis and the level of connexin43 in astrocytes
Abstract
Introduction: Acute cerebral ischemia is caused by an insufficient blood supply to brain tissue. Oxygen therapy, which is able to aid diffusion to reach the ischemic region, has been regarded as a possible treatment for cerebral ischemia. Recent animal and pilot clinical studies have reported that normobaric hyperoxia (NBO) showed neuroprotective effects if started soon after the onset of stroke. However, little is known about the role and mechanism of NBO treatment in astrocytes. Connexin43, one of the main gap junction proteins in astrocytes, is extremely sensitive to hypoxia and oxidative stress after cerebral ischemia.
Aims: In the present study, we used sutures to develop an ischemia/reperfusion model in rats to mimic clinical recanalization and investigated the role of connexin43 in NBO-treated stroke rats, as well as the underlying mechanism of NBO therapy.
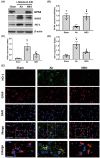
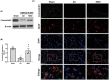
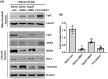
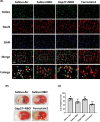
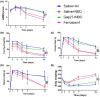
Results: Normobaric hyperoxia treatment maintained the homeostasis of oxidoreductases: glutathione peroxidase 4 (GPX4) and NADPH oxidase 4 (two important oxidoreductases) and rescued the ischemia/reperfusion-induced downregulation of connexin43 protein in astrocytes. Furthermore, NBO treatment attenuated cerebral ischemia-induced cytochrome c release from mitochondria and was involved in neuroprotective effects by regulating the GPX4 and connexin43 pathway, using Ferrostatin-1 (an activator of GPX4) or Gap27 (an inhibitor of connexin43).
Conclusions: This study showed the neuroprotective effects of NBO treatment by reducing oxidative stress and maintaining the level of connexin43 in astrocytes, which could be used for the clinical treatment of ischemic stroke.
Keywords: acute ischemic stroke; astrocyte; gap junction connexin43; glutathione peroxidase 4 (GPX4); normobaric hyperoxia; oxidative stress.
© 2022 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures
Similar articles
-
Inhibition of Connexin43 hemichannels with Gap19 protects cerebral ischemia/reperfusion injury via the JAK2/STAT3 pathway in mice.Brain Res Bull. 2019 Mar;146:124-135. doi: 10.1016/j.brainresbull.2018.12.009. Epub 2018 Dec 26. Brain Res Bull. 2019. PMID: 30593877
-
Normobaric hyperoxia retards the evolution of ischemic brain tissue toward infarction in a rat model of transient focal cerebral ischemia.Neurol Res. 2016 Jan;38(1):75-9. doi: 10.1080/01616412.2015.1135558. Epub 2016 Feb 18. Neurol Res. 2016. PMID: 27078693
-
Normobaric hyperoxia slows blood-brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia.Stroke. 2015 May;46(5):1344-1351. doi: 10.1161/STROKEAHA.114.008599. Epub 2015 Mar 24. Stroke. 2015. PMID: 25804925 Free PMC article.
-
Normobaric oxygen treatment in acute ischemic stroke: a clinical perspective.Med Gas Res. 2016 Oct 14;6(3):147-153. doi: 10.4103/2045-9912.191360. eCollection 2016 Jul-Sep. Med Gas Res. 2016. PMID: 27867482 Free PMC article. Review.
-
Cocktail treatment, a promising strategy to treat acute cerebral ischemic stroke?Med Gas Res. 2016 Apr 4;6(1):33-38. doi: 10.4103/2045-9912.179343. eCollection 2016 Mar. Med Gas Res. 2016. PMID: 27826421 Free PMC article. Review.
Cited by
-
Role of mitochondrial metabolism in ischemic stroke and natural products intervention.Mol Biol Rep. 2025 Jun 7;52(1):568. doi: 10.1007/s11033-025-10667-0. Mol Biol Rep. 2025. PMID: 40481903 Review.
-
The neuroprotective effects of normobaric oxygen therapy after stroke.CNS Neurosci Ther. 2024 Jul;30(7):e14858. doi: 10.1111/cns.14858. CNS Neurosci Ther. 2024. PMID: 39009510 Free PMC article. Review.
-
Magnetic resonance imaging quantitative assessment of corticospinal tract damage in basal ganglia infarction.Medicine (Baltimore). 2024 Oct 25;103(43):e40300. doi: 10.1097/MD.0000000000040300. Medicine (Baltimore). 2024. PMID: 39470499 Free PMC article.
-
Normobaric hyperoxia alleviates complement C3-mediated synaptic pruning and brain injury after intracerebral hemorrhage.CNS Neurosci Ther. 2024 Mar;30(3):e14694. doi: 10.1111/cns.14694. CNS Neurosci Ther. 2024. PMID: 38532579 Free PMC article.
-
Neuronal Zinc Transporter ZnT3 Modulates Cerebral Ischemia-Induced Blood-Brain Barrier Disruption.Aging Dis. 2023 Oct 18;15(6):2727-2741. doi: 10.14336/AD.2023.1011. Aging Dis. 2023. PMID: 37962463 Free PMC article.
References
-
- Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11‐21. - PubMed
-
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723‐1731. - PubMed
-
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467‐477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

