A double-blind, randomized, placebo-controlled phase 2 trial evaluating the selective dihydroorotate dehydrogenase inhibitor vidofludimus calcium in relapsing-remitting multiple sclerosis
- PMID: 35698927
- PMCID: PMC9268865
- DOI: 10.1002/acn3.51574
A double-blind, randomized, placebo-controlled phase 2 trial evaluating the selective dihydroorotate dehydrogenase inhibitor vidofludimus calcium in relapsing-remitting multiple sclerosis
Abstract
Objective: Inhibition of dihydroorotate dehydrogenase suppresses magnetic resonance imaging brain lesions and disease activity in multiple sclerosis but has limiting tolerability. We assessed the safety and efficacy of vidofludimus calcium, a novel, selective dihydroorotate dehydrogenase inhibitor, in patients with relapsing-remitting multiple sclerosis.
Methods: This double-blind, 24 weeks, placebo-controlled, phase 2 trial (EMPhASIS) enrolled patients 18-55 years with relapsing-remitting multiple sclerosis. Eligible patients were randomly assigned (1:1:1) to once-daily vidofludimus calcium (30 mg or 45 mg) or placebo. The primary endpoint was the cumulative number of combined unique active lesions to week 24 between vidofludimus calcium 45 mg and placebo (clinicalTrials.gov number NCT03846219; EudraCT 2018-001896-19).
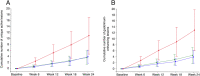
Results: After 24 weeks, the mean cumulative number of combined unique active lesions was 6.4 (95% CI: 2.8-13.9) with placebo compared to 2.4 (95% CI: 1.1-4.9) with vidofludimus calcium 45 mg (rate ratio 0.38, 95% CI: 0.22-0.64; p = 0.0002); the rate ratio between vidofludimus calcium 30 mg and placebo was 0.30 (95% CI: 0.17-0.53; p < 0.0001). Treatment-emergent adverse events occurred in 30 (44%) of patients assigned placebo and 60 (43%) of patients assigned vidofludimus calcium. Serious adverse events occurred in one (1%) assigned placebo and two (1%) assigned vidofludimus calcium. No increased incidence of infectious, hepatic, or renal treatment-emergent adverse events or serious adverse events was observed.
Interpretation: Treatment with vidofludimus calcium led to a reduction in new magnetic resonance imaging lesions in patients with relapsing-remitting multiple sclerosis and was well tolerated with a favorable safety profile. Assessment in longer, larger trials is justified.
© 2022 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
RJF reports personal fees from AB Science, Biogen, Celgene, EMD Serono, Genentech, Genzyme, Immunic AG, Janssen Novartis, Sanofi, and TG Therapeutics; clinical trial contracts from Biogen, Novartis, and Sanofi.
HW reports grants and personal fees from AbbVie, Biogen, Merck Serono, and Sanofi Genzyme; personal fees from Actelion, Alexion, Evgen, F. Hoffmann‐La Roche, Gemeinnützige Hertie‐Stiftung, Immunic, Lundbeck, MedDay Pharmaceuticals, Novartis, Roche Pharma AG, Teva, and WebMD Global; and grants from Deutsche Forschungsgesellschaft (DFG), Else Kröner Fresenius Foundation, European Union, Fresenius Foundation, German Ministry for Education and Research (BMBF), GlaxoSmithKline, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, NRW Ministry of Education and Research, PML Consortium, RE Children's Foundation, and Swiss MS Society, outside the submitted work.
CW is a partner at Lycalis sprl and reports compensation for his organization for consulting from BMS, Celgene, Desitin, Immunic, Merck KGaA, Novartis, Roche, Synthon, Teva, and Viatris; and for speaking from Synthon and Viatris.
NdS has received honoraria from Biogen‐ Idec, Genzyme, Immunic, Merck, Novartis, Roche, Celgene and Teva for consulting services, speaking, and travel support. He serves on advisory boards for Merck, Novartis, Biogen‐Idec, Immunic, Roche, and Genzyme, and he has received research grant support from the Italian MS Society.
JS received honoraria for participation in advisory boards, consultancy, and lecturing from Biogen, BMS, Immunic, Merck, Novartis, Roche, and Sanofi.
VG: reports grants/personal fees from Medpace, PPD, PRA Health Sciences, PSI, Roche, Sanofi, and Verum.
KR is the president elect of the Polish Neurological Society.
PSB reports no declaration of interests.
NT reports no declaration of interests.
IS reports no declaration of interests.
AM is a shareholder and employee of trial sponsor, and a holder of patents for the drug under investigation.
Figures
References
-
- Rückemann K, Fairbanks LD, Carrey EA, et al. Leflunomide inhibits pyrimidine de novo synthesis in mitogen‐stimulated T‐lymphocytes from healthy humans. J Biol Chem. 1998;273(34):21682‐21691. - PubMed
-
- Löffler M, Klein A, Hayek‐Ouassini M, Knecht W, Konrad L. Dihydroorotate dehydrogenase mRNA and protein expression analysis in Normal and drug‐resistant cells. Nucleosides Nucleotides Nucleic Acids. 2004;23:1281‐1285. - PubMed
-
- Jameson SC. Maintaining the norm: T‐cell homeostasis. Nat Rev Immunol. 2002;2(8):547‐556. - PubMed
-
- Fairbanks LD, Bofill M, Ruckemann K, Simmonds HA. Importance of ribonucleotide availability to proliferating T‐lymphocytes from healthy humans: disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J Biol Chem. 1995;270(50):29682‐29689. - PubMed
-
- O'Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of Oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293‐1303. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
