Sex-Based Differences in Outcomes Following Peripheral Artery Revascularization: Insights From VOYAGER PAD
- PMID: 35699170
- PMCID: PMC9238670
- DOI: 10.1161/JAHA.121.024655
Sex-Based Differences in Outcomes Following Peripheral Artery Revascularization: Insights From VOYAGER PAD
Abstract
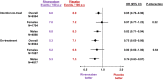
Background Despite high female prevalence of peripheral artery disease (PAD), little is known about sex-based outcomes after lower extremity revascularization (LER) for symptomatic PAD. The effects of rivaroxaban according to sex following LER have not been fully reported. Methods and Results In VOYAGER PAD (Vascular Outcomes Study of ASA [acetylsalicylic acid] Along with Rivaroxaban in Endovascular or Surgical Limb Revascularization for Peripheral Artery Disease), low-dose rivaroxaban versus placebo on a background of aspirin reduced the composite primary efficacy outcome of cardiovascular and limb events in patients with PAD undergoing LER. Unplanned index limb revascularization was prespecified and prospectively ascertained. The primary safety outcome was Thrombolysis in Myocardial Infarction major bleeding. Analyses of outcomes and treatment effects by sex were performed using Cox proportional hazards models. Among 6564 randomly assigned patients followed for a median of 28 months, 1704 (26.0%) were women. Among patients administered placebo, women were at similar risk for the primary efficacy outcome (hazard ratio [HR], 0.90; [95% CI, 0.74-1.09]; P=0.29) as men, while female sex was associated with a trend toward higher risk of unplanned index limb revascularization (HR, 1.18; [95% CI, 1.00-1.40]; P=0.0499). Irrespective of sex, effects of rivaroxaban were consistent for the primary efficacy outcome (P-interaction=0.22), unplanned index limb revascularization (P-interaction=0.64), and bleeding (P-interaction=0.61). Women were more likely than men to discontinue study treatment (HR, 1.13; [95% CI, 1.03-1.25]; P=0.0099). Conclusions Among >1700 women with PAD undergoing LER, women and men were at similar risk for the primary outcome, but a trend for greater risk of unplanned index limb revascularization among women was observed. Effects of rivaroxaban were consistent by sex, though women more often discontinued treatment. Better understanding of sex-based outcomes and treatment adherence following LER is needed. Registration URL: http://clinicaltrials.gov; Unique identifier: NCT02504216.
Keywords: outcomes; peripheral artery disease; revascularization; sex.
Figures


References
-
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 - DOI - PubMed
-
- Subherwal S, Patel MR, Kober L, Peterson ED, Bhatt DL, Gislason GH, Olsen AM, Jones WS, Torp‐Pedersen C, Fosbol EL. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol. 2015;22:317–325. doi: 10.1177/2047487313519344 - DOI - PubMed
-
- Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, et al. Low‐density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial. Circulation. 2018;137:338–350. doi: 10.1161/CIRCULATIONAHA.117.032235 - DOI - PubMed
-
- Bonaca MP, Scirica BM, Creager MA, Olin J, Bounameaux H, Dellborg M, Lamp JM, Murphy SA, Braunwald E, Morrow DA. Vorapaxar in patients with peripheral artery disease: results from TRA2°P‐TIMI 50. Circulation. 2013;127:1522–1529, 1529.e1‐6 - PubMed
-
- Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Goto S, Ohman EM, Elbez Y, Sritara P, Baumgartner I, et al. Statin therapy and long‐term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864–2872. doi: 10.1093/eurheartj/ehu080 - DOI - PMC - PubMed

