Low-Density Lipoprotein Cholesterol Attributable Cardiovascular Disease Risk Is Sex Specific
- PMID: 35699189
- PMCID: PMC9238661
- DOI: 10.1161/JAHA.121.024248
Low-Density Lipoprotein Cholesterol Attributable Cardiovascular Disease Risk Is Sex Specific
Abstract
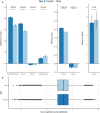
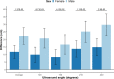
Background Epidemiological studies show that women are generally at lower risk for cardiovascular disease than men. Here, we investigated the sex-specific differential effect of genetically increased low-density lipoprotein cholesterol (LDL-C) on cardiovascular disease (CVD) and other lipid-associated diseases. Methods and Results This is a 2-sample Mendelian randomization study that uses individual participant data from 425 043 participants from the UK Biobank, including 229 279 female participants. An 80-variant LDL-C weighted genetic score was generated. Linear and logistic regression models with interactions were used to identify differences between sex-specific LDL-C effects on lipids, carotid-intima media thickness, and multiple cardiovascular outcomes such as CVD, ischemic heart disease, peripheral artery disease, heart failure, aortic valve disease, type 2 diabetes, atrial fibrillation, and aortic aneurysm and dissection. After correction for multiple testing, we observed that the genetically increased LDL-C effect on CVD events was sex specific: per SD genetically increased LDL-C, female participants had a higher LDL-C increase but an attenuated CVD risk increase compared with male participants (LDL-C: female participants 0.71 mmol/L, 95% CI, 0.70-0.72 and male participants 0.57 mmol/L, 95% CI, 0.56-0.59. P for interaction: 5.03×10-60; CVD: female participants: odds ratio [OR], 1.32; 95% CI 1.24-1.40 and male participants: OR, 1.52; 95% CI, 1.46-1.58. P for interaction: 9.88×10-5). We also observed attenuated risks for ischemic heart disease and (nominally for) heart failure in female participants, and genetically increased LDL-C results in higher risk for aortic valve disease in female participants compared with male participants. Genetically increased LDL-C was also associated with an attenuated carotid-intima media thickness increase in female participants. We did not observe other significant attenuations. Sensitivity analyses with an unweighted genetic score and sex-specific weighted genetic scores showed similar results. Conclusions We found that genetically increased LDL-C has a sex-specific differential effect on the risk for cardiovascular disease, ischemic heart disease, heart failure, and aortic valve stenosis. Our observations provide evidence that LDL-C might be a less important determinant of CVD in women compared with men, suggesting that male patients might benefit more from LDL-C targeted therapies for CVD management than female patients and warranting investigations into the sex-specific relative contribution of risk factors for CVD.
Keywords: cardiovascular disease; genetics; risk factor; sex‐differences.
Figures


Similar articles
-
Genetic Determinants of Lipids and Cardiovascular Disease Outcomes: A Wide-Angled Mendelian Randomization Investigation.Circ Genom Precis Med. 2019 Dec;12(12):e002711. doi: 10.1161/CIRCGEN.119.002711. Epub 2019 Nov 22. Circ Genom Precis Med. 2019. PMID: 31756303 Free PMC article.
-
Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis.JAMA. 2014 Nov 5;312(17):1764-71. doi: 10.1001/jama.2014.13959. JAMA. 2014. PMID: 25344734 Free PMC article.
-
Risk scores of common genetic variants for lipid levels influence atherosclerosis and incident coronary heart disease.Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2233-9. doi: 10.1161/ATVBAHA.113.301236. Epub 2013 Jun 13. Arterioscler Thromb Vasc Biol. 2013. PMID: 23766260
-
Omega-3 Fatty Acids and Cardiovascular Disease: An Updated Systematic Review.Evid Rep Technol Assess (Full Rep). 2016 Aug;(223):1-1252. doi: 10.23970/AHRQEPCERTA223. Evid Rep Technol Assess (Full Rep). 2016. PMID: 30307737
-
Residual Atherosclerotic Cardiovascular Disease Risk: Focus on Non-High-Density Lipoprotein Cholesterol.J Cardiovasc Pharmacol Ther. 2023 Jan-Dec;28:10742484231189597. doi: 10.1177/10742484231189597. J Cardiovasc Pharmacol Ther. 2023. PMID: 37641208 Review.
Cited by
-
Women, lipids, and atherosclerotic cardiovascular disease: a call to action from the European Atherosclerosis Society.Eur Heart J. 2023 Oct 14;44(39):4157-4173. doi: 10.1093/eurheartj/ehad472. Eur Heart J. 2023. PMID: 37611089 Free PMC article.
-
Dose-Response Associations of Lipid Traits With Coronary Artery Disease and Mortality.JAMA Netw Open. 2024 Jan 2;7(1):e2352572. doi: 10.1001/jamanetworkopen.2023.52572. JAMA Netw Open. 2024. PMID: 38241044 Free PMC article.
-
Evaluating the effect of green tea intake on cardiovascular diseases: A Mendelian randomization study in European and East Asian populations.Medicine (Baltimore). 2024 Jul 19;103(29):e38977. doi: 10.1097/MD.0000000000038977. Medicine (Baltimore). 2024. PMID: 39029022 Free PMC article.
-
Beyond LDL cholesterol: remnant cholesterol is associated with cardiometabolic risk factors in children.BMC Med. 2025 Jan 21;23(1):28. doi: 10.1186/s12916-025-03859-9. BMC Med. 2025. PMID: 39838462 Free PMC article.
-
Cardiovascular contributions to dementia: Examining sex differences and female-specific factors.Alzheimers Dement. 2025 Aug;21(8):e70610. doi: 10.1002/alz.70610. Alzheimers Dement. 2025. PMID: 40851413 Free PMC article. Review.
References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update a report from the American Heart Association WRITING GROUP MEMBERS on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2020;141:139–596. doi: 10.1161/CIR.0000000000000757 - DOI - PubMed
-
- Swiger KJ, Martin SS, Blaha MJ, Toth PP, Nasir K, Michos ED, Gerstenblith G, Blumenthal RS, Jones SR. Narrowing sex differences in lipoprotein cholesterol subclasses following mid‐life: the very large database of lipids (VLDL‐10B). J Am Heart Assoc. 2014;3. doi: 10.1161/JAHA.114.000851 - DOI - PMC - PubMed
-
- Cangemi R, Romiti GF, Campolongo G, Ruscio E, Sciomer S, Gianfrilli D, Raparelli V. Gender related differences in treatment and response to statins in primary and secondary cardiovascular prevention: the never‐ending debate. Pharmacol Res. 2017;117:148–155. doi: 10.1016/j.phrs.2016.12.027 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

