PML Alternative Splice Products Differentially Regulate HAdV Productive Infection
- PMID: 35699431
- PMCID: PMC9431499
- DOI: 10.1128/spectrum.00785-22
PML Alternative Splice Products Differentially Regulate HAdV Productive Infection
Abstract
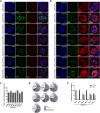
Promyelocytic leukemia nuclear bodies (PML-NBs) were considered to maintain antiviral capacity, as these spherical complexes are antagonized by viruses. Actual work provides evidence, that PML-NB-associated factors might also be beneficial for distinct viral processes indicating why genomes and replication centers of nuclear replicating viruses are often found juxtaposed to PML-NBs. Several early HAdV proteins target PML-NBs, such as E4orf3 that promotes redistribution into track-like structures. PML-associated dependency factors that enhance viral gene expression, such as Sp100A remain in the nuclear tracks while restrictive factors, such as Daxx, are inhibited by either proteasomal degradation or relocalization to repress antiviral functions. Here, we did a comprehensive analysis of nuclear PML isoforms during HAdV infection. Our results show cell line specific differences as PML isoforms differentially regulate productive HAdV replication and progeny production. Here, we identified PML-II as a dependency factor that supports viral progeny production, while PML-III and PML-IV suppress viral replication. In contrast, we identified PML-I as a positive regulator and PML-V as a restrictive factor during HAdV infection. Solely PML-VI was shown to repress adenoviral progeny production in both model systems. We showed for the first time, that HAdV can reorganize PML-NBs that contain PML isoforms other then PML-II. Intriguingly, HAdV was not able to fully disrupt PML-NBs composed out of the PML isoforms that inhibit viral replication, while PML-NBs composed out of PML isoforms with beneficial influence on the virus formed tracks in all examined cells. In sum, our findings clearly illustrate the crucial role of PML-track formation in efficient viral replication. IMPORTANCE Actual work provides evidence that PML-NB-associated factors might also be beneficial for distinct viral processes indicating why genomes and replication centers of nuclear replicating viruses are often found juxtaposed to PML-NBs. Alternatively spliced PML isoforms I-VII are expressed from one single pml gene containing nine exons and their transcription is tightly controlled and stimulated by interferons and p53. Several early HAdV proteins target PML-NBs, such as E4orf3, promoting redistribution into track-like structures. Our comprehensive studies indicate a diverging role of PML isoforms throughout the course of productive HAdV infection in either stably transformed human lung (H1299) or liver (HepG2) cells, in which we observed a multivalent regulation of HAdV by all six PML isoforms. PML-I and PML-II support HAdV-mediated track formation and efficient formation of viral replication centers, thus promoting HAdV productive infection. Simultaneously, PML-III, -IV,-V, and -VI antagonize viral gene expression and particle production.
Keywords: HAdV; PML-NB; SUMO; human adenovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

