Evaluation of Diagnostic Strategies for Identifying SARS-CoV-2 Infection in Clinical Practice: a Systematic Review and Compliance with the Standards for Reporting Diagnostic Accuracy Studies Guideline (STARD)
- PMID: 35699441
- PMCID: PMC9430610
- DOI: 10.1128/spectrum.00300-22
Evaluation of Diagnostic Strategies for Identifying SARS-CoV-2 Infection in Clinical Practice: a Systematic Review and Compliance with the Standards for Reporting Diagnostic Accuracy Studies Guideline (STARD)
Abstract
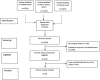
We aimed to review strategies for identifying SARS-CoV-2 infection before the availability of molecular test results, and to assess the reporting quality of the studies identified through the application of the STARD guideline. We screened 3,821 articles published until 30 April 2021, of which 23 met the inclusion criteria: including at least two diagnostic variables, being designed for use in clinical practice or in a public health context and providing diagnostic accuracy rates. Data extraction and application of STARD criteria were performed independently by two researchers and discrepancies were discussed with a third author. Most of the studies (16, 69.6%) included symptomatic patients with suspected infection, six studies (26.1%) included patients already diagnosed and one study (4.3%) included individuals with close contact to a COVID-positive patient. The main variables considered in the studies, which included symptomatic patients, were imaging and demographic characteristics, symptoms, and lymphocyte count. The values for area under the receiver operating characteristic curve (AUC)ranged from 53-97.4. Seven studies (30.4%) validated the diagnostic model in an independent sample. The average number of STARD criteria fulfilled was 17.6 (maximum, 27 and minimum, 5). High diagnostic accuracy values are shown when more than one diagnostic variable is considered, mainly imaging and demographic characteristics, symptoms, and lymphocyte count. This could offer the potential to identify individuals with SARS-CoV-2 infection with high accuracy when molecular testing is not available. However, external validation for developed models and evaluations in populations as similar as possible to those in which they will be applied is urgently needed. IMPORTANCE According to this review, the inclusion of more than one diagnostic test in the diagnostic process for COVID-19 infection shows high diagnostic accuracy values. Imaging characteristics, patients' symptoms, demographic characteristics, and lymphocyte count were the variables most frequently included in the diagnostic models. However, developed models should be externally validated before reaching conclusions on their utility in practice. In addition, it is important to bear in mind that the test should be evaluated in populations as similar as possible to those in which it will be applied in practice.
Keywords: COVID-19; SARS-CoV-2; STARD; diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yanes-Lane M, Winters N, Fregonese F, Bastos M, Perlman-Arrow S, Campbell JR, Menzies D. 2020. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: a systematic review and meta-analysis. PLoS One 15:e0241536. doi: 10.1371/journal.pone.0241536. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous