Bacterial Necromass Is Rapidly Metabolized by Heterotrophic Bacteria and Supports Multiple Trophic Levels of the Groundwater Microbiome
- PMID: 35699474
- PMCID: PMC9431026
- DOI: 10.1128/spectrum.00437-22
Bacterial Necromass Is Rapidly Metabolized by Heterotrophic Bacteria and Supports Multiple Trophic Levels of the Groundwater Microbiome
Abstract
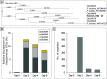
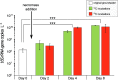
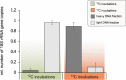
Pristine groundwater is a highly stable environment with microbes adapted to dark, oligotrophic conditions. Input events like heavy rainfalls can introduce the excess particulate organic matter, including surface-derived microorganisms, thereby disturbing the groundwater microbiome. Some surface-derived bacteria will not survive this translocation, leading to an input of necromass to the groundwater. Here, we investigated the effects of necromass addition to the microbial community in fractured bedrock groundwater, using groundwater mesocosms as model systems. We followed the uptake of 13C-labeled necromass by the bacterial and eukaryotic groundwater community quantitatively and over time using a complementary protein-stable and DNA-stable isotope probing approach. Necromass was rapidly depleted in the mesocosms within 4 days, accompanied by a strong decrease in Shannon diversity and a 10-fold increase in bacterial 16S rRNA gene copy numbers. Species of Flavobacterium, Massilia, Rheinheimera, Rhodoferax, and Undibacterium dominated the microbial community within 2 days and were identified as key players in necromass degradation, based on a 13C incorporation of >90% in their peptides. Their proteomes comprised various proteins for uptake and transport functions and amino acid metabolization. After 4 and 8 days, the autotrophic and mixotrophic taxa Nitrosomonas, Limnohabitans, Paucibacter, and Acidovorax increased in abundance with a 13C incorporation between 0.5% and 23%. Likewise, eukaryotes assimilated necromass-derived carbon either directly or indirectly. Our data point toward a fast and exclusive uptake of labeled necromass by a few specialists followed by a concerted action of groundwater microorganisms, including autotrophs presumably fueled by released, reduced nitrogen and sulfur compounds generated during necromass degradation. IMPORTANCE Subsurface microbiomes provide essential ecosystem services, like the generation of drinking water. These ecosystems are devoid of light-driven primary production, and microbial life is adapted to the resulting oligotrophic conditions. Modern groundwater is most vulnerable to anthropogenic and climatic impacts. Heavy rainfalls, which will increase with climate change, can result in high surface inputs into shallow aquifers by percolation or lateral flow. These inputs include terrestrial organic matter and surface-derived microbes that are not all capable to flourish in aquatic subsurface habitats. Here, we investigated the response of groundwater mesocosms to the addition of bacterial necromass, simulating event-driven surface input. We found that the groundwater microbiome responds with a rapid bloom of only a few primary degraders, followed by the activation of typical groundwater autotrophs and mixotrophs, as well as eukaryotes. Our results suggest that this multiphase strategy is essential to maintain the balance of the groundwater microbiome to provide ecosystem services.
Keywords: AquaDiva; groundwater; metaproteomics; necromass; stable isotope probing; subsurface; surface input.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Akob DM, Küsel K. 2011. Where microorganisms meet rocks in the Earth’s Critical Zone. Biogeosciences 8:3531–3543. doi: 10.5194/bg-8-3531-2011. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

